The amount of heat in the SI system is measured. Methods and means of measuring the amount of heat
Heat- the energy transferred from a hotter body to a less heated one by direct contact or by radiation.
Temperature is a measure of the intensity of molecular motion.
The amount of heat possessed by a body at a given temperature depends on its mass; For example, at the same temperature, more heat is contained in a large cup of water than in a small one, and in a bucket of cold water it can be more than in a cup of hot water(although the temperature of the water in the bucket is lower).
Heat plays an important role in human life, including in the functioning of his body. Part of the chemical energy contained in food is converted into heat, due to which the body temperature is maintained near 37 ° C. The heat balance of the human body also depends on temperature. environment, and people are forced to spend a lot of energy on heating residential and industrial premises in winter and cooling them in summer. Most of this energy is supplied by thermal engines, such as boiler plants and steam turbines of power plants that run on fossil fuels (coal, oil) and generate electricity.
Until the end of the 18th century. heat was considered a material substance, believing that the temperature of a body is determined by the amount of<калорической жидкости>, or<теплорода>. Later, B. Rumford, J. Joule and other physicists of that time, through ingenious experiments and reasoning, refuted<калорическую>theory, proving that heat is weightless and can be obtained in any quantity simply due to mechanical movement. Heat in itself is not a substance - it is just the energy of the movement of its atoms or molecules. It is this understanding of heat that modern physics adheres to.
In this article, we will look at how heat and temperature are related and how these quantities are measured. The subject of our discussion will also be the following questions: the transfer of heat from one part of the body to another; heat transfer in vacuum (a space that does not contain matter); the role of heat in the modern world.
Heat and temperature
The amount of thermal energy in a substance cannot be determined by observing the movement of each of its molecules separately. On the contrary, only by studying the macroscopic properties of a substance, one can find the characteristics of the microscopic motion of many molecules averaged over a certain period of time. The temperature of a substance is the average indicator of the intensity of the movement of molecules, the energy of which is thermal energy substances.
One of the most familiar, but also the least accurate ways of estimating temperature is by touch. Touching an object, we judge whether it is hot or cold, focusing on our feelings. Of course, these sensations depend on the temperature of our body, which brings us to the concept of thermal equilibrium - one of the most important in measuring temperature.
Thermal equilibrium
Thermal equilibrium between bodies A and B
Obviously, if two bodies A and B are pressed tightly against each other, then, after touching them after a sufficiently long time, we will notice that their temperature is the same. In this case, bodies A and B are said to be in thermal equilibrium with each other. However, bodies, generally speaking, do not have to be in contact for thermal equilibrium to exist between them - it is enough that their temperatures are the same. This can be verified using the third body C, first bringing it into thermal equilibrium with body A, and then comparing the temperatures of bodies C and B. Body C here plays the role of a thermometer. In a strict formulation, this principle is called the zeroth law of thermodynamics: if bodies A and B are in thermal equilibrium with a third body C, then these bodies are also in thermal equilibrium with each other. This law underlies all methods of measuring temperature.
Temperature measurement

Temperature scales

thermometers
Thermometers based on electrical effects
If we want to conduct accurate experiments and calculations, then such temperature ratings as hot, warm, cool, cold are not enough - we need a graduated temperature scale. There are several such scales, and the freezing and boiling points of water are usually taken as reference points. The four most common scales are shown in the figure. The centigrade scale, according to which the freezing point of water corresponds to 0 °, and the boiling point to 100 °, is called the Celsius scale named after A. Celsius, a Swedish astronomer who described it in 1742. It is believed that the Swedish naturalist K. Linnaeus first applied this scale. Now the Celsius scale is the most common in the world. The Fahrenheit temperature scale, in which the freezing and boiling points of water correspond to extremely uncomfortable numbers of 32 and 212 °, was proposed in 1724 by G. Fahrenheit. The Fahrenheit scale is widely used in English-speaking countries, but it is hardly used in the scientific literature. To convert Celsius temperature (°C) to Fahrenheit temperature (°F), there is a formula °F = (9/5)°C + 32, and for the reverse translation - the formula °C = (5/9) (°F- 32).
Both scales - both Fahrenheit and Celsius - are very inconvenient when conducting experiments in conditions where the temperature drops below the freezing point of water and is expressed as a negative number. For such cases, absolute temperature scales were introduced, which are based on extrapolation to the so-called absolute zero - the point at which molecular motion should stop. One of them is called the Rankin scale, and the other is called the absolute thermodynamic scale; temperatures are measured in degrees Rankine (°R) and kelvins (K). Both scales start at absolute zero, and the freezing point of water corresponds to 491.7 ° R and 273.16 K. The number of degrees and kelvins between the freezing and boiling points of water on the Celsius scale and the absolute thermodynamic scale is the same and equal to 100; for the Fahrenheit and Rankine scales, it is also the same, but equal to 180. Celsius degrees are converted to kelvins using the formula K \u003d ° C + 273.16, and degrees Fahrenheit are converted to Rankine degrees using the formula ° R \u003d ° F + 459.7.
The operation of devices designed to measure temperature is based on various physical phenomena associated with a change in the thermal energy of a substance - changes in electrical resistance, volume, pressure, radiative characteristics, thermoelectric properties. One of the simplest and most familiar temperature measuring instruments is the glass thermometer shown in the figure. The ball c in the lower part of the thermometer is placed in the medium or pressed against the object whose temperature is to be measured, and depending on whether the ball receives heat or gives off, expands or contracts and its column rises or falls in the capillary. If the thermometer is pre-calibrated and equipped with a scale, then you can directly find out the body temperature.
Another device whose action is based on thermal expansion is bimetal thermometershown in the figure. Its main element is a spiral plate of two soldered metals with different coefficients of thermal expansion. When heated, one of the metals expands more than the other, the spiral twists and turns the arrow relative to the scale. Such devices are often used to measure indoor and outdoor air temperature, but they are not suitable for determining local temperature.
Local temperature is usually measured using a thermocouple, which is two wires of dissimilar metals soldered at one end. When such a junction is heated, an EMF arises at the free ends of the wires, usually a few millivolts. Thermocouples are made from different metal pairs: iron and constantan, copper and constantan, chromel and alumel. Their thermo-EMF changes almost linearly with temperature over a wide temperature range.
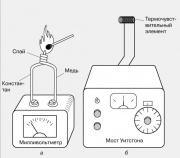
Another thermoelectric effect is also known - the dependence of the resistance of a conductive material on temperature. It underlies the operation of electrical resistance thermometers, one of which is shown in the figure. The resistance of a small temperature sensing element (thermocouple) - usually coils of thin wire - is compared with the resistance of a calibrated variable resistor using a Wheatstone bridge. The output instrument can be graduated directly in degrees.
Optical pyrometers are used to measure the temperature of hot bodies that emit visible light. In one version of this device, the light emitted by the body is compared with the radiation of an incandescent lamp filament placed in the focal plane of the binoculars through which the emitting body is viewed. Electric current, heating the lamp filament, is changed until, when visually comparing the glow of the filament and the body, it is found that thermal equilibrium has been established between them. The scale of the device can be graduated directly in units of temperature.
Technical advances recent years allowed to create new temperature sensors. For example, in cases where particularly high sensitivity is needed, instead of a thermocouple or a conventional resistance thermometer, a semiconductor device is used - thermistor. Dyes and liquid crystals that change their phase state are also used as thermal converters, especially in cases where the body surface temperature varies over a wide range. Finally, infrared thermography is used, in which an infrared image of an object is obtained in conditional colors, where each color corresponds to a certain temperature. This method of temperature measurement finds the widest application - from medical diagnostics before checking the thermal insulation of the premises.
Measuring the amount of heat
water calorimeter
The thermal energy (amount of heat) of a body can be measured directly with a so-called calorimeter; A simple version of such a device is shown in the figure. It is a carefully insulated closed vessel, equipped with devices for measuring the temperature inside it, and sometimes filled with a working fluid of known properties, such as water. To measure the amount of heat in a small heated body, it is placed in a calorimeter and waiting for the system to come into thermal equilibrium. The amount of heat transferred to the calorimeter (more precisely, to the water filling it) is determined by the increase in water temperature.
The amount of heat released during a chemical reaction, such as combustion, can be measured by placing a small<бомбу>. AT<бомбе>a sample is located, to which electric wires for ignition are connected, and the corresponding amount of oxygen. After the sample completely burns out and thermal equilibrium is established, it is determined how much the temperature of the water in the calorimeter has increased, and hence the amount of heat released.
Heat units
Heat is a form of energy and therefore must be measured in units of energy. In the international SI system, the unit of energy is the joule (J). It is also allowed to use off-system units of the amount of heat - calories: an international calorie is 4.1868 J, a thermochemical calorie is 4.1840 J. In foreign laboratories, research results are often expressed using the so-called. A 15-degree calorie equal to 4.1855 J. The off-system British Thermal Unit (BTU) is falling into disuse: BTU avg = 1.055 J.
Heat sources
The main sources of heat are chemical and nuclear reactions, as well as various energy conversion processes. Examples of chemical reactions with the release of heat are combustion and the breakdown of food components. Almost all the heat received by the Earth is provided by nuclear reactions occurring in the depths of the Sun. Mankind has learned how to obtain heat with the help of controlled processes of nuclear fission, and now it is trying to use thermonuclear fusion reactions for the same purpose. Other types of energy can also be converted into heat, such as mechanical work and electrical energy. It is important to remember that thermal energy (like any other) can only be converted into another form, but cannot be obtained.<из ничего>, nor destroy. This is one of the basic principles of the science called thermodynamics.
Thermodynamics
Thermodynamics is the science of the relationship between heat, work and matter. Modern ideas about these relationships were formed on the basis of the works of such great scientists of the past as Carnot, Clausius, Gibbs, Joule, Kelvin, and others. Thermodynamics explains the meaning of the heat capacity and thermal conductivity of a substance, the thermal expansion of bodies, and the heat of phase transitions. This science is based on several experimentally established laws - principles.
Heat and properties of substances
Various substances have different ability to accumulate thermal energy; it depends on their molecular structure and density. The amount of heat required to raise the temperature of a unit mass of a substance by one degree is called its specific heat capacity. The heat capacity depends on the conditions in which the substance is located. For example, to heat one gram of air in a balloon by 1 K, more heat is required than to heat it in the same way in a sealed vessel with rigid walls, since part of the energy imparted to the balloon is spent on expanding the air, and not on heating it. Therefore, in particular, the heat capacity of gases is measured separately at constant pressure and at constant volume.
With an increase in temperature, the intensity of the chaotic movement of molecules increases - most substances expand when heated. The degree of expansion of a substance with an increase in temperature by 1 K is called coefficient of thermal expansion.
In order for a substance to pass from one phase state to another, for example, from solid to liquid (and sometimes immediately to gaseous), it must receive a certain amount of heat. If heated solid, then its temperature will rise until it begins to melt; until the melting is completed, the temperature of the body will remain constant despite the heat input. The amount of heat required to melt a unit mass of a substance is called the heat of fusion. If you continue to supply heat, then the molten substance will heat up to a boil. The amount of heat required to vaporize a unit mass of a liquid at a given temperature is called the heat of vaporization.
The role of heat and its use
Scheme of operation of a steam turbine power plant
Refrigeration cycle diagram
Global heat transfer processes are not reduced to heating the Earth by solar radiation. Massive convection currents in the atmosphere determine daily changes in weather conditions throughout the globe. Temperature differences in the atmosphere between the equatorial and polar regions, together with the Coriolis forces due to the rotation of the Earth, lead to the appearance of continuously changing convection currents, such as trade winds, jet streams, and warm and cold fronts.
The transfer of heat (due to thermal conductivity) from the molten core of the Earth to its surface leads to volcanic eruptions and the appearance of geysers. In some regions, geothermal energy is used for space heating and power generation.
Warmth is an indispensable participant in almost all production processes. We will mention the most important of them, such as the smelting and processing of metals, the operation of engines, food production, chemical synthesis, oil refining, the manufacture of a variety of items - from bricks and dishes to cars and electronic devices.
Many industrial productions and transport, as well as thermal power plants, could not work without heat engines - devices that convert heat into useful work. Examples of such machines are compressors, turbines, steam, gasoline, and jet engines.
One of the best known heat engines is the steam turbine, which implements part of the Rankine cycle used in modern power plants. A simplified diagram of this cycle is shown in the figure. The working fluid - water - is converted into superheated steam in a steam boiler heated by burning fossil fuels (coal, oil or natural gas). Steam high
Gasovik - industrial gas equipment Directory of GOST, SNiP, PB Units of physical quantities, physico-chemical concepts, ratios, composition and characteristics of gases
Units of measurement for temperature and quantity of heat
The basic unit of temperature measurement was the degree of the International Temperature Scale, which practically corresponds to the degree Celsius. This value is equal to 1/100 of the temperature interval between 0 and 100 ° C, i.e. between the melting points of ice and boiling water at a pressure of 760 mm Hg. Art.
Absolute temperature is the temperature measured from absolute zero, i.e. from -273.16 ° C, and measured in degrees Kelvin (° K). The Kelvin degree is the same as the Celsius degree. Therefore, the absolute temperature is expressed in degrees centigrade as follows:
T, °K = t, °C + 273.16
In the SI system, the unit of temperature is the Kelvin. It is allowed to express the practical results of temperature measurements using the degree Celsius along with the degree Kelvin, depending on the origin (position of zero) on the scale.
Example: 250 ±5 °С = 523.16 ±5 °K.
In the SI system, work, energy, and heat are measured in joules (J). Sometimes a larger and more convenient unit for practical purposes is used - a kilojoule (kJ), equal to 1000 J. A unit of work in SI is the work done by a force of 1 N at a displacement of 1 m. Energy - physical quantity showing how much work the body can do.
Calories and kilocalories can be used as off-system heat units. A calorie is the amount of heat required to raise the temperature of 1 gram of water by 1°C (19.5 to 20.5°C).
1 cal (calorie) = 4.1868 J;
1 kcal (kilocalorie) \u003d 1000 cal \u003d 4186.8 J \u003d 4.187 kJ;
1 Mcal (megacalorie) \u003d 10 6 cal \u003d 4.1868 MJ;
1 Gcal (gigacalorie) \u003d 10 9 cal \u003d 4186.8 MJ.
For comparison, when evaluating fuel, the so-called conditional heat is used, the calorific value of which for calculation is assumed to be conditionally equal to 7 Mcal/kg or 7 Gcal/t. In such cases, one speaks, respectively, of 1 kg or 1 ton of standard fuel (t.c.f.).
Heat plays an important role in human life, including in the functioning of his body. Part of the chemical energy contained in food is converted into heat, due to which the body temperature is maintained near 37
° C. The heat balance of the human body also depends on the ambient temperature, and people are forced to spend a lot of energy on heating residential and industrial premises in winter and cooling them in summer. Most of this energy is supplied by heat engines, such as boiler plants and steam turbines of power plants that run on fossil fuels (coal, oil) and generate electricity.Until the end of the 18th century. heat was considered a material substance, believing that the temperature of a body is determined by the amount of "caloric liquid" or "caloric" contained in it. Later, B. Rumford, J. Joule and other physicists of that time, through ingenious experiments and reasoning, refuted the "caloric" theory, proving that heat is weightless and it can be obtained in any quantity simply due to mechanical movement. Heat itself is not a substance it is just the energy of the movement of its atoms or molecules. It is this understanding of heat that modern physics adheres to. see also PHYSICS.
In this article, we will look at how heat and temperature are related and how these quantities are measured. The subject of our discussion will also be the following questions: the transfer of heat from one part of the body to another; heat transfer in vacuum (a space that does not contain matter); the role of heat in the modern world.
HEAT AND TEMPERATURE The amount of thermal energy in a substance cannot be determined by observing the movement of each of its molecules separately. On the contrary, only by studying the macroscopic properties of matter, one can find the characteristics of the microscopic motion of many molecules averaged over a certain period of time. The temperature of a substance is an average indicator of the intensity of movement of molecules, the energy of which is the thermal energy of a substance.One of the most familiar, but also the least accurate ways to assess temperature by touch. Touching an object, we judge whether it is hot or cold, focusing on our feelings. Of course, these sensations depend on the temperature of our body, which brings us to the concept of thermal equilibrium, one of the most important in measuring temperature.
Thermal balance. Obviously, if two bodies A and B (Fig. 1) tightly pressed against each other, then, having touched them after a sufficiently long time, we will notice that their temperature is the same. In this case, the bodies are said to be A and B are in thermal equilibrium with each other. However, bodies, generally speaking, do not have to be in contact for thermal equilibrium to exist between them, it is enough that their temperatures are the same. This can be verified using the third body C , bringing it first into thermal equilibrium with the body A , and then comparing the temperatures of the bodies C and b. Body C here plays the role of a thermometer. In a strict formulation, this principle is called the zeroth law of thermodynamics: if bodies A and B are in thermal equilibrium with a third body C, then these bodies are also in thermal equilibrium with each other. This law underlies all methods of measuring temperature.Temperature measurement. If we want to conduct accurate experiments and calculations, then such temperature ratings as hot, warm, cool, cold are not enough we need a graduated temperature scale. There are several such scales, and the freezing and boiling points of water are usually taken as reference points. The four most common scales are shown in fig. 2. Centigrade scale, according to which the freezing point of water corresponds to 0° , and the boiling point is 100° , is called the Celsius scale named after A. Celsius, a Swedish astronomer who described it in 1742. It is believed that the Swedish naturalist K. Linney was the first to use this scale. Now the Celsius scale is the most common in the world. The Fahrenheit temperature scale, in which the freezing and boiling points of water correspond to the extremely uncomfortable numbers 32 and 212° , was proposed in 1724 by G. Fahrenheit. The Fahrenheit scale is widely used in English-speaking countries, but it is hardly used in the scientific literature. To convert temperature to Celsius (° C) to Fahrenheit temperature (° F) there is a formula° F = (9/5) ° C + 32, and for the reverse translation formula°C = (5/9)(°F - 32). Both scales, both Fahrenheit and Celsius, are very inconvenient when conducting experiments in conditions where the temperature drops below the freezing point of water and is expressed as a negative number. For such cases, absolute temperature scales have been introduced, which are based on extrapolation to the so-called absolute zero, the point at which molecular motion must stop. One of them is called the Rankin scale, and the other is called the absolute thermodynamic scale; temperatures are measured in degrees Rankine (° R) and kelvins (K). Both scales start at absolute zero and the freezing point of water is 491.7° R and 273.16 K. The number of degrees and kelvins between the freezing and boiling points of water on the Celsius scale and the absolute thermodynamic scale is the same and equal to 100; for the Fahrenheit and Rankine scales, it is also the same, but equal to 180. Celsius degrees are converted to kelvins using the formula K \u003d° C + 273.16, and degrees Fahrenheit to degrees Rankine using the formula°R = °F + 459.7. The operation of devices designed to measure temperature is based on various physical phenomena associated with a change in the thermal energy of a substance, changes in electrical resistance, volume, pressure, radiative characteristics, and thermoelectric properties. One of the simplest and most familiar instruments for measuring temperature is the mercury-in-glass thermometer shown in fig. 3, a. The ball with mercury in the lower part of the thermometer is placed in the medium or pressed against the object whose temperature they want to measure, and depending on whether the ball receives heat or gives off, the mercury expands or contracts and its column rises or falls in the capillary. If the thermometer is pre-calibrated and equipped with a scale, then you can directly find out the body temperature.Another device whose operation is based on thermal expansion is the bimetal thermometer shown in fig.
3, b. Its main element spiral plate of two soldered metals with different coefficients of thermal expansion. When heated, one of the metals expands more than the other, the spiral twists and turns the arrow relative to the scale. Such devices are often used to measure indoor and outdoor air temperature, but they are not suitable for determining local temperature.Local temperature is usually measured using a thermocouple, which is two wires of dissimilar metals soldered at one end (Fig.
4, a). When such a junction is heated, an emf arises at the free ends of the wires, usually a few millivolts. Thermocouples are made from different metal pairs: iron and constantan, copper and constantan, chromel and alumel. Their thermo-EMF changes almost linearly with temperature over a wide temperature range.Another thermoelectric effect is also known - the dependence of the resistance of a conductive material on temperature. It underlies the operation of electrical resistance thermometers, one of which is shown in Fig.
4, b. The resistance of a small temperature sensitive element (thermal sensor) usually coils of thin wire is compared with the resistance of a calibrated variable resistor using a Wheatstone bridge. The output instrument can be graduated directly in degrees.Optical pyrometers are used to measure the temperature of incandescent bodies that emit visible light. In one version of this device, the light emitted by the body is compared with the radiation of an incandescent lamp filament placed in the focal plane of the binoculars through which the emitting body is viewed. The electric current that heats the lamp filament is changed until a visual comparison of the glow of the filament and the body reveals that thermal equilibrium has been established between them. The scale of the device can be graduated directly in units of temperature.
Technical advances in recent years have made it possible to create new temperature sensors. For example, in cases where particularly high sensitivity is needed, a semiconductor device, the thermistor, is used instead of a thermocouple or conventional resistance thermometer. Dyes and liquid crystals that change their phase state are also used as thermal converters, especially in cases where the body surface temperature varies over a wide range. Finally, infrared thermography is used, in which an infrared image of an object is obtained in conditional colors, where each color corresponds to a certain temperature. This method of temperature measurement finds the widest application from medical diagnostics to checking the thermal insulation of rooms. see also PHYSICS OF THE SOLID STATE; LIQUID CRYSTAL.
Measurement of the amount of heat. The thermal energy (amount of heat) of a body can be measured directly with a so-called calorimeter; A simple version of such a device is shown in Fig. 5. This is a carefully insulated closed vessel, equipped with devices for measuring the temperature inside it and sometimes filled with a working fluid with known properties, such as water. To measure the amount of heat in a small heated body, it is placed in a calorimeter and waiting for the system to come into thermal equilibrium. The amount of heat transferred to the calorimeter (more precisely, to the water filling it) is determined by the increase in water temperature.The amount of heat released during a chemical reaction, such as combustion, can be measured by placing a small "bomb" in the calorimeter. The "bomb" contains a sample, to which electrical wires are connected for ignition, and the corresponding amount of oxygen. After the sample burns out completely and thermal equilibrium is established, it is determined how much the temperature of the water in the calorimeter has increased, and hence the amount of heat released. see also CALORIMETRY.
Heat units. Heat is a form of energy and therefore must be measured in units of energy. In the international SI system, the unit of energy is the joule (J). It is also allowed to use non-systemic units of the amount of heat calories: an international calorie is 4.1868 J, a thermochemical calorie is 4.1840 J. In foreign laboratories, research results are often expressed using the so-called. A 15-degree calorie equal to 4.1855 J. The off-system British Thermal Unit (BTU) is falling into disuse: BTU avg = 1.055 J. The main sources of heat are chemical and nuclear reactions, as well as various energy conversion processes. Examples of chemical reactions with the release of heat are combustion and the breakdown of food components. Almost all the heat received by the Earth is provided by nuclear reactions occurring in the depths of the Sun. Mankind has learned how to obtain heat with the help of controlled processes of nuclear fission, and now it is trying to use thermonuclear fusion reactions for the same purpose. Other types of energy can also be converted into heat, such as mechanical work and electrical energy. It is important to remember that thermal energy (like any other) can only be transformed into another form, but it can neither be obtained "out of nothing" nor destroyed. This is one of the basic principles of the science called thermodynamics. THERMODYNAMICS Thermodynamics is the science of the relationship between heat, work and matter. Modern ideas about these relationships were formed on the basis of the works of such great scientists of the past as Carnot, Clausius, Gibbs, Joule, Kelvin, and others. Thermodynamics explains the meaning of the heat capacity and thermal conductivity of a substance, the thermal expansion of bodies, and the heat of phase transitions. This science is based on several experimentally established laws principles.Beginnings of thermodynamics. The zeroth law of thermodynamics formulated above introduces the concepts of thermal equilibrium, temperature and thermometry. The first law of thermodynamics is a statement of key importance for all science as a whole: energy can neither be destroyed nor obtained "out of nothing", so the total energy of the Universe is a constant value. In its simplest form, the first law of thermodynamics can be stated as follows: the energy that the system receives, minus the energy that it gives up, is equal to the energy remaining in the system. At first glance, this statement seems obvious, but not in such, for example, situations like the combustion of gasoline in the cylinders of an automobile engine: here the energy received is chemical, the energy given off is mechanical (work), and the energy remaining in the system is thermal.So, it is clear that energy can change from one form to another and that such transformations are constantly taking place in nature and technology. More than a hundred years ago, J. Joule proved this for the case of the conversion of mechanical energy into thermal energy using the device shown in fig. 6, a. In this device, descending and rising weights rotated a shaft with blades in a calorimeter filled with water, as a result of which the water was heated. Precise measurements allowed Joule to determine that one calorie of heat is equivalent to 4.186 J of mechanical work. The device shown in fig.
6, b, was used to determine the thermal equivalent of electrical energy.The first law of thermodynamics explains many common phenomena. For example, it becomes clear why it is impossible to cool the kitchen with an open refrigerator. Let's assume that we have thermally insulated the kitchen from the environment. Energy is continuously supplied to the system through the power wire of the refrigerator, but the system does not give off any energy. Thus, its total energy increases, and the kitchen becomes warmer: just touch the tubes of the heat exchanger (condenser) on the back of the refrigerator, and you will understand its uselessness as a "cooling" device. But if these pipes were brought out of the system (for example, out of the window), then the kitchen would give out more energy than it received, i.e. would be cooled, and the refrigerator worked as a window air conditioner.
The first law of thermodynamics is a law of nature that precludes the creation or destruction of energy. However, it says nothing about how the processes of energy transfer proceed in nature. Thus, we know that a hot body will heat a cold one if these bodies are brought into contact. But can a cold body by itself transfer its heat reserve to a hot one? Last Opportunity categorically rejected by the second law of thermodynamics.
The first law also excludes the possibility of creating an engine with a coefficient useful action(efficiency) more than 100% (similar
" eternal " the engine could give off more energy for an arbitrarily long time than it consumes). It is impossible to build an engine even with an efficiency equal to 100%, since some part of the energy supplied to it must necessarily be lost by it in the form of less useful thermal energy. So, the wheel will not spin indefinitely without energy supply, because due to friction in the bearings, the energy of mechanical movement will gradually turn into heat until the wheel stops.The tendency to turn "useful" work into less useful energy heat can be compared with another process that occurs when two vessels containing different gases are connected. After waiting long enough, we find in both vessels a homogeneous mixture of gases nature acts in such a way that the order of the system decreases. The thermodynamic measure of this disorder is called entropy, and the second law of thermodynamics can be formulated differently: processes in nature always proceed in such a way that the entropy of the system and its environment increases. Thus, the energy of the Universe remains constant, while its entropy is continuously growing.
Heat and properties of substances. Different substances have different ability to store thermal energy; it depends on their molecular structure and density. The amount of heat required to raise the temperature of a unit mass of a substance by one degree is called its specific heat. The heat capacity depends on the conditions in which the substance is located. For example, to heat one gram of air in a balloon by 1 K, more heat is required than to heat it in the same way in a sealed vessel with rigid walls, since part of the energy imparted to the balloon is spent on expanding the air, and not on heating it. Therefore, in particular, the heat capacity of gases is measured separately at constant pressure and at constant volume.With an increase in temperature, the intensity of the chaotic movement of molecules increases most substances expand when heated. The degree of expansion of a substance with an increase in temperature by 1 K is called the coefficient of thermal expansion.
In order for a substance to pass from one phase state to another, for example, from solid to liquid (and sometimes immediately to gaseous), it must receive a certain amount of heat. If a solid body is heated, its temperature will rise until it begins to melt; until the melting is completed, the temperature of the body will remain constant, despite the supply of heat. The amount of heat required to melt a unit mass of a substance is called the heat of fusion. If you continue to supply heat, then the molten substance will heat up to a boil. The amount of heat required to vaporize a unit mass of a liquid at a given temperature is called the heat of vaporization.
Molecular-kinetic theory. The molecular kinetic theory explains the macroscopic properties of a substance by considering at the microscopic level the behavior of the atoms and molecules that make up this substance. In this case, a statistical approach is used and some assumptions are made about the particles themselves and the nature of their motion. Thus, molecules are considered to be solid balls, which in gaseous media are in continuous chaotic motion and run considerable distances from one collision to another. Collisions are considered elastic and occur between particles whose size is small and the number is very large. None of the real gases corresponds exactly to this model, but most gases are quite close to it, which is the reason for the practical value of the molecular kinetic theory.Based on these ideas and using a statistical approach, Maxwell derived the distribution of the velocities of gas molecules in a limited volume, which was later named after him. This distribution is presented graphically in fig. 7 for a given mass of hydrogen at temperatures of 100 and 1000
° C. The ordinate represents the number of molecules moving at the speed indicated on the abscissa. The total number of particles is equal to the area under each curve and is the same in both cases. It can be seen from the graph that most of the particles have velocities close to some average value, and only a small number of them have very high or low velocities. The average velocities at these temperatures lie in the range 2000-3000 m/s, i.e. very large.A large number of such rapidly moving gas molecules acts with a completely measurable force on the surrounding bodies. The microscopic forces with which numerous gas molecules hit the walls of the vessel add up to a macroscopic quantity called pressure. When energy is supplied to a gas (temperature rises), the average kinetic energy of its molecules increases, gas particles hit the walls more often and harder, the pressure rises, and if the walls are not completely rigid, then they stretch and the gas volume increases. Thus, the microscopic statistical approach underlying the molecular kinetic theory makes it possible to explain the phenomenon of thermal expansion that we have discussed.
Another result of molecular kinetic theory is a law that describes the properties of a gas that satisfies the requirements listed above. This is the so-called equation of state ideal gas connects the pressure, volume and temperature of one mole of gas and has the form of equality
PV = RT where P pressure, V volume, T temperature, and R universal gas constant equal to (8.31441± 0.00026) J/(mol H TO). see also MOLECULAR-KINETIC THEORY; THERMODYNAMICS. HEAT TRANSFER Heat transfer is the process of transferring heat within a body or from one body to another, due to a temperature difference. The intensity of heat transfer depends on the properties of the substance, temperature difference and obeys the experimentally established laws of nature. To create efficient heating or cooling systems, various engines, power plants, thermal insulation systems, you need to know the principles of heat transfer. In some cases, heat exchange is undesirable (thermal insulation of melting furnaces, spaceships, etc.), while in others it should be as large as possible (steam boilers, heat exchangers, kitchen utensils).There are three main types of heat transfer: conduction, convection and radiant heat transfer.
Thermal conductivity. If there is a temperature difference inside the body, then thermal energy passes from its hotter part to its colder one. This type of heat transfer, due to thermal movements and collisions of molecules, is called thermal conductivity; at enough high temperatures in solids it can be observed visually. So, when a steel rod is heated from one end in the flame of a gas burner, thermal energy is transferred through the rod, and a glow spreads at a certain distance from the heated end (less and less intense with distance from the place of heating).The intensity of heat transfer due to thermal conductivity depends on the temperature gradient, i.e. relations
D T/D x temperature difference at the ends of the rod to the distance between them. It also depends on the cross-sectional area of the rod (in m 2 ) and the thermal conductivity of the material[ in corresponding units W/(m Ch K) ] . The relationship between these quantities was derived by the French mathematician J. Fourier and has the following form: where q heat flow, k thermal conductivity coefficient, and A cross-sectional area. This relationship is called Fourier's law of heat conduction; the minus sign in it indicates that heat is transferred in the opposite direction to the temperature gradient.It follows from the Fourier law that the heat flux can be reduced by reducing one of the quantities the thermal conductivity coefficient, area or temperature gradient. For a building in winter conditions, the latter values are practically constant, and therefore, in order to maintain the desired temperature in the room, it remains to reduce the thermal conductivity of the walls, i.e. improve their thermal insulation.
The table shows the thermal conductivity coefficients of some substances and materials. The table shows that some metals conduct heat much better than others, but all of them are much better heat conductors than air and porous materials.
|
THERMAL CONDUCTIVITY OF SOME SUBSTANCES AND MATERIALS |
|
|
Substances and materials |
Thermal conductivity, W / (m × K) |
| Aluminum | |
| Bronze | |
| Bismuth | |
| Tungsten | |
| Iron | |
| Gold | |
| Cadmium | |
| Magnesium | |
| Copper | |
| Arsenic | |
| Nickel | |
| Platinum | |
| Mercury | |
| Lead | |
| Zinc | |
|
Other materials |
|
| Asbestos | |
| Concrete | |
| Air | |
| Eider down (loose) | |
| Tree nut) | |
| Magnesia (MgO) | |
| Sawdust | |
| Rubber (sponge) | |
| Mica | |
| Glass | |
| Carbon (graphite) | |
The thermal and electrical resistance of many substances decreases sharply as the temperature drops below the temperature of liquid helium (1.8 K). This phenomenon, called superconductivity, is used to improve the efficiency of many devices, from microelectronic devices to power lines and large electromagnets. see also SUPERCONDUCTIVITY.
Convection. As we have already said, when heat is applied to a liquid or gas, the intensity of the movement of molecules increases, and as a result, the pressure increases. If a liquid or gas is not limited in volume, then they expand; the local density of the liquid (gas) becomes less, and due to the buoyancy (Archimedean) forces, the heated part of the medium moves up (which is why the warm air in the room rises from the batteries to the ceiling). This phenomenon is called convection. In order not to waste the heat of the heating system for nothing, you need to use modern heaters that provide forced air circulation.The convective heat flow from the heater to the heated medium depends on the initial velocity of the molecules, density, viscosity, thermal conductivity and heat capacity, and the medium; the size and shape of the heater are also very important. The ratio between the corresponding quantities obeys Newton's law
q = hA( T W- TҐ ), where q heat flow (measured in watts), A surface area of the heat source (in m 2), T W and T temperature of the source and its environment (in kelvins). Convective heat transfer coefficient h depends on the properties of the medium, the initial velocity of its molecules, and also on the shape of the heat source, and is measured in units of W/(m 2 h TO).Value
h is not the same for cases when the air around the heater is stationary (free convection) and when the same heater is in the air flow (forced convection). In simple cases of fluid flow through a pipe or flow around a flat surface, the coefficient h can be calculated theoretically. However, it has not yet been possible to find an analytical solution to the problem of convection for a turbulent flow of a medium. Turbulence is a complex movement of a liquid (gas), chaotic on a scale that significantly exceeds the molecular ones.If a heated (or, conversely, cold) body is placed in a stationary medium or in a flow, then convective currents and a boundary layer are formed around it. The temperature, pressure, and velocity of molecules in this layer play an important role in determining the coefficient of convective heat transfer.
Convection must be considered in the design of heat exchangers, air conditioning systems, high speed aircraft and many other applications. In all such systems, heat conduction takes place simultaneously with convection, both between solids and in their environment. At elevated temperatures Radiant heat transfer can also play a significant role.
Radiant heat transfer. The third type of heat transfer radiant heat transfer differs from heat conduction and convection in that heat in this case can be transferred through a vacuum. Its similarity with other methods of heat transfer is that it is also due to the temperature difference. Thermal radiation is one of the types electromagnetic radiation. Other types of it radio wave, ultraviolet and gamma radiation occur in the absence of a temperature difference.On fig. 8 shows the dependence of the energy of thermal (infrared) radiation on the wavelength. Thermal radiation can be accompanied by the emission of visible light, but its energy is small compared to the radiation energy of the invisible part of the spectrum.
The intensity of heat transfer by heat conduction and convection is proportional to temperature, and the radiant heat flux is proportional to the fourth power of temperature and obeys the Stefan Boltzmann law
Residential and office spaces are often heated with small electric heat emitters; the reddish glow of their spirals is visible thermal radiation close to the edge of the infrared part of the spectrum. The room is heated by heat, which is carried mainly by the invisible, infrared part of the radiation. In night vision devices
^ The camera uses a thermal radiation source and an IR-sensitive receiver that allows you to see in the dark.The Sun is a powerful emitter of thermal energy; it heats the Earth even at a distance of 150 million km. The intensity of solar radiation, recorded year after year by stations located in many parts of the globe, is approximately 1.37 W
/ m 2 . Solar energy is the source of life on Earth. Searches are being made for ways to use it most effectively. Solar panels have been created to heat houses and generate electricity for domestic needs. THE ROLE OF HEAT AND ITS USE Global heat transfer processes are not reduced to heating the Earth by solar radiation. Massive convection currents in the atmosphere determine daily changes in weather conditions throughout the globe. Temperature differences in the atmosphere between the equatorial and polar regions, together with the Coriolis forces due to the rotation of the Earth, lead to the appearance of continuously changing convection currents, such as trade winds, jet streams, and warm and cold fronts. see also CLIMATE; METEOROLOGY AND CLIMATOLOGY.The transfer of heat (due to thermal conductivity) from the molten core of the Earth to its surface leads to volcanic eruptions and the appearance of geysers. In some regions, geothermal energy is used for space heating and power generation.
Heat is an indispensable participant in almost all production processes. We will mention the most important of them, such as the smelting and processing of metals, the operation of engines, the production of food products, chemical synthesis, oil refining, the manufacture of a wide variety of objects from bricks and dishes to cars and electronic devices.
Many industrial productions and transport, as well as thermal power plants, could not work without heat engines - devices that convert heat into useful work. Examples of such machines are compressors, turbines, steam, gasoline and jet engines.
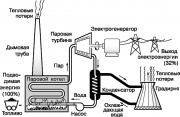
One of the most famous heat engines is the steam turbine, which implements part of the Rankine cycle used in modern power plants. A simplified diagram of this cycle is shown in fig. 9. The working fluid water is converted into superheated steam in a steam boiler heated by burning fossil fuels (coal, oil or natural gas). Steam high pressure rotates the shaft of a steam turbine, which drives a generator that generates electricity. The exhaust steam condenses when cooled by running water, which absorbs some of the heat not used in the Rankine cycle. Next, the water is fed into the cooling tower (cooling tower), from where part of the heat is released into the atmosphere. The condensate is pumped back to the steam boiler and the whole cycle is repeated.
All processes in the Rankine cycle illustrate the principles of thermodynamics described above. In particular, according to the second law, part of the energy consumed by the power plant must be dissipated in the environment in the form of heat. It turns out that about 68% of the energy originally contained in fossil fuels is lost in this way. A noticeable increase in the efficiency of the power plant could be achieved only by raising the temperature of the steam boiler (which is limited by the heat resistance of materials) or by lowering the temperature of the medium where the heat goes, i.e. atmosphere.
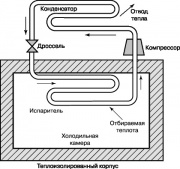
Another thermodynamic cycle having great importance in our Everyday life, is the Rankin vapor-compressor refrigeration cycle, the diagram of which is shown in fig. 10. In refrigerators and household air conditioners, energy is supplied from outside to provide it. The compressor increases the temperature and pressure of the working substance of the refrigerator freon, ammonia or carbon dioxide. The superheated gas is fed into the condenser, where it is cooled and condensed, giving off heat to the environment. The liquid leaving the condenser nozzles passes through the throttling valve into the evaporator, and part of it evaporates, which is accompanied by a sharp drop in temperature. The evaporator takes heat from the refrigerator chamber, which heats the working fluid in the nozzles; this liquid is supplied by the compressor to the condenser, and the cycle repeats again.
The refrigeration cycle shown in fig. 10 can also be used in a heat pump. Such heat pumps in summer give off heat to hot atmospheric air and condition the room, and in winter, on the contrary, they take heat from cold air and heat the room.
Nuclear reactions are an important source of heat for purposes such as power generation and transportation. In 1905, A. Einstein showed that mass and energy are related by the relation
E=mc2 , i.e. can pass into each other. speed of light c very large: 300 thousand km/ With. This means that even a small amount of matter can provide a huge amount of energy. So, from 1 kg of fissile material (for example, uranium), it is theoretically possible to obtain energy, which for 1000 days of continuous operation is provided by a power plant with a capacity of 1 MW. see also ATOM STRUCTURE; FURNACES AND FURNACES TECHNOLOGY; ELECTROMAGNETIC RADIATION; HEAT EXCHANGER; TURBINE; UNITS OF MEASUREMENT OF PHYSICAL QUANTITIES.LITERATURE Zemansky M. Temperatures very high and very low. M., 1968Paul R. Mechanics, acoustics and the doctrine of heat. M., 1971
Smorodinsky Ya.A. Temperature. M., 1981
Fan J. Machines, energy and entropy. M., 1986
Atkins P.V. Order and disorder in nature. M., 1987
Liquid calorimeters
This type of calorimeter, the most widely used in technology, is simple in design and easy to maintain. The amount of heat produced by an externally induced reaction is first transferred to the reaction vessel (in which the reaction took place) and then to the liquid bath. The liquid in the bath is continuously agitated by means of an impeller, an elevating screw or pumps, which speeds up the equalization of temperatures. The bath is thermally insulated (shielded) from the environment as much as possible. The temperature change of the liquid bath is a measure of the amount of heat detected. The heat capacity of the masses to be heated must not be too high in order to ensure a sufficient change in temperature and so that the measurement process does not last too long (due to which heat losses increase).
Figure Device of a liquid calorimeter.
With high requirements for constancy of ambient conditions, it is possible to place the entire calorimeter in another bath and stabilize the temperature in it with high accuracy using a control loop. This is necessary in the first place in cases where it is required to conduct an experiment at temperatures that differ significantly from the ambient temperature.
For analysis at low temperatures (down to about -150°C), liquid nitrogen is used as a cooling medium. In this case, it is necessary to pay attention to the fact that when changing them, frost from the surrounding humid air does not precipitate on the samples or sample vessels, since its layer can affect the measurement process. To avoid this, when the calorimeter is open, the sample and the sample container are purged with cold nitrogen gas.
Metal body calorimeters
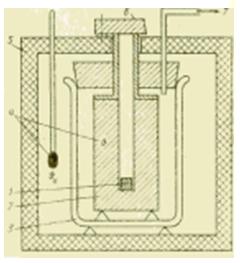
Figure Scheme of the device of a metal calorimeter.
If it is required to carry out calorimetric studies in a wider temperature range, then liquid calorimeters are no longer suitable. In metal body calorimeters suitable for this purpose, the amount of heat transferred is taken up by a metal block (silver, copper, aluminium) which is usually at ambient temperature. Such a calorimeter is mainly intended for determining the specific heat capacity c, J / (kg * K), liquid and solid substances.
The sample is first cooled outside the calorimeter in refrigeration plant or heated in a furnace and, after reaching a steady state, lowered (dropped) into a hole in a metal block. According to the method of operation, such a device is called a free-fall calorimeter, and according to the nature of the thermodynamic processes in it, it is sometimes called a displacement calorimeter.
The amount of heat transferred during this mixing from the sample (with parameters m1, c1, ) to the metal block (m2, c2, ) causes a measurable change in the temperature of the block. This makes it possible to determine the usually unknown value of the specific heat capacity of the sample for ideal conditions(in the absence of heat exchange with the environment) from the expression:
The metal block itself is located in an evacuated Dewar vessel, and sometimes in a liquid bath. In the latter case, to obtain the heat capacity of the calorimeter Ck, the heat capacity of the bath Cw must be added to the heat capacity of the metal block C2:
CK=C2+CW=c2m2+cWmW .
CALORIMETRIC METHODS OF MEASUREMENT
Most often, calorimeters are used in the mode of constant environmental conditions. This applies primarily to most combustion calorimeters, in which the reaction time is very short. While the temperature of the internal parts of the calorimeter changes due to the reaction, the ambient air temperature remains constant. In many cases, a temperature-controlled bath is used as the environment in order to avoid influence on the measured value of external interference - temperature fluctuations in the room, radiation, drafts, etc.
The advantage of this measuring scheme is the relatively low cost of equipment, which can be used to perform the predominant part of the calorimetric measurements. The main disadvantage should be considered the heat exchange of the calorimeter with the environment, which complicates the interpretation of the results. This method of measurement is always called isoperibol (diathermic). In any case, it cannot be called isothermal, the essence of which lies in the fact that the temperature of the calorimeter remains constant during the course of the reaction, as, for example, calorimeters designed to measure phase transformations.
adiabatic method
If it is possible to exclude heat exchange with the environment, i.e., to ensure the adiabatic flow of the process, then the experiment and interpretation of the results are simplified, and the measurement result is more accurate, since there is no need to continuously record the temperature change and calculate corrections. In addition, in this case, a somewhat greater rise in temperature in the calorimeter vessel can be tolerated; for non-adiabatic devices, this is unacceptable due to an increase in heat losses.
To avoid heat exchange between the calorimeter vessel and its immediate surroundings (usually the liquid bath), the temperature of the bath must be constantly corrected according to temperature changes within the vessel. With the help of an electronic controller (tracking circuit) it is possible to constantly maintain the difference of these temperatures practically equal to zero. This increases the cost of measuring equipment depending on the required measurement accuracy.
Hardware elements must be fast and stable for a long time (have minimal drift). The dead zone of the tracking control loop should be in the range from ±10-3 to ±10-5 K. As measuring devices, any fast-response electric contact thermometers can be used, which, when included in the bridge circuit, give an impulse to the controller to change the heating power. Heating is carried out either by means of an electrical resistance coil or directly in a liquid bath, which acts as a heating resistor due to weak dissociation (so-called electrolytic heating). This second method is practically inertialess. The result can be obtained using already existing means for electrical temperature measurement or using an additionally installed liquid thermometer (Beckmann).
The adiabatic measurement method is suitable for studying mainly slow processes and thermal effects. With rapid changes in the amount of heat (in combustion calorimeters), the inertia of temperature equalization has such an unfavorable effect that even the accuracy of conventional non-adiabatic methods is not achieved. However, by providing a low heat capacity of the heating elements and temperature sensors and by intensive mixing of the bath liquid, it is possible to obtain small values of various time constants (reduce the inertia).
Compensation method
Using differential or dual calorimeters based on the principle of compensation, it is possible to largely eliminate external influences on the measurement process. Two identical calorimetric vessels with identical accessories are placed in an environment under the same conditions. In one vessel, the investigated process with a thermal effect occurs, and the other vessel is heated with the help of a servo control system in such a way that the heat loss to the environment for both vessels is the same. Therefore, the input heating power can be put in direct proportion to the amount of heat released during the process under study. In this case, the experimental task of measurement goes into another area and is reduced to a very accurate determination of the supplied electric heating power (W * s, J):
The differential calorimeter is used, in particular, under adiabatic ambient conditions, especially when very small or very slow changes in the amount of heat are to be expected. In endothermic processes, it is sufficient to have one calorimetric vessel. The heat input is controlled in such a way that the temperature in the vessel remains the same all the time (isothermal method). The disadvantage of differential calorimeters is the high cost of equipment and measuring instruments.
COMBUSTION CALORIMETERS
The fuel used in heat and power facilities is examined in order to determine its calorific value H (J/kg). This indicator is needed to determine efficiency factors, study efficiency and calculate the energy consumed in various installations, as well as for optimal control of the combustion process. Significant fluctuations in the composition of combustible components often necessitate continuous determination of the calorific value.
When a substance is completely burned, a certain amount of heat Q (heat of combustion) is released. If we divide it by the mass m (or by the volume under normal conditions Vn), we get the (specific) heat of combustion:
Depending on the state of the combustion products, two types of calorific value are distinguished: higher H0 and lower H, which are also called the heat of combustion and calorific value. When determining the net calorific value of Ni, the water formed during chemical reactions must be in a vapor state. The difference between both heats H0 - Ni corresponds to the heat of vaporization of condensed water (index KO - condensate) r, which is equal to 2.441 MJ / kg.
For solid and liquid fuels, the resulting amount of water can be determined on the basis of elemental analysis, and when burning gaseous fuels, by measuring the amount of condensate.
In industrial furnaces, the temperature of the combustion products always exceeds the boiling point of water. Therefore, only the net calorific value Ni is usually of interest, since the heat of condensation of water cannot be used.
Combustion Calorimeters for Solids and Liquids
For fast combustion processes, a special form of a liquid calorimeter has been developed - the so-called Berthelot calorimetric bomb (Fig. 3).
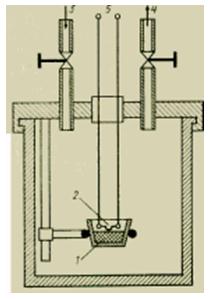
Figure The device of a calorimetric bomb.
The combustion of a small, precisely measured amount of a substance occurs at a constant volume in a sealed bomb in an atmosphere of the purest possible oxygen at a pressure of ~30 atm (3 MPa). The filled bomb is placed in the liquid bath of the calorimeter, which absorbs the released heat of combustion.
Solids usually pressed into small briquettes (tablets) and weighed very accurately. It is advisable to mix poorly burning substances with well-burning liquids with a known heat of combustion (for example, benzoic acid). Liquid substances are placed in cups (boats) made of platinum or quartz or in small plastic capsules. On the cover, bolted to the body of the bomb, there are all the devices necessary for research: valves for supplying oxygen and removing combustion products, sample holders and an electric igniter. Ignition is carried out by supplying electricity to a thin platinum wire. The heat supplied for ignition must be accurately measured so that it can be taken into account when deciphering the results of the experiment. In a calorimetric bomb, the highest calorific value H0 is determined. During verification, the thermal equivalent of the calorimeter Ck is determined by burning a reference substance (for example, benzoic acid) or using an electric heating device.
Combustion calorimeter for gaseous substances
To determine the heat of combustion of gaseous media, there are various methods. All of them, in contrast to the calorimetric bomb for solid and liquid substances, are based on continuous measurement. The measurement principle used is quite simple. The test gas is continuously burned in a burner at a constant pressure. All heat released during combustion is absorbed either by the flow of the cooling medium in the heat exchanger (wet or heat-exchange calorimeter), or by mixing the combustion products with the air flow at a known flow rate (dry or mixing calorimeter). Usually determine the net calorific value Hu. To determine the gross calorific value H0, it is necessary to condense the water vapor (index KO) contained in the flue gases. Knowing the mass flow rates and the temperature difference at the inlet (index e) and outlet (index a) of the calorimeter, it is possible to calculate the corresponding calorific value using the heat balance equation.
The required gas preparation is basically the same in all gas calorimeters. Before combustion, the gas (index G) is first cleaned from solid mechanical impurities (in the filter) and moistened (to saturation with moisture, 100%), and then brought to the specified values of preliminary pressure (using a pressure reducing valve) and temperature of the cooling medium (index K) . The air required for combustion (index L) is also humidified and brought to the temperature of the cooling medium.
Depending on the required accuracy and allowable instrumentation costs, some of these conditions may not be met. Calorimeters should be verified with a reference gas (e.g. hydrogen) to determine the deviation from the equation for the ideal state of the calorimeter. For a heat-exchange (wet) calorimeter, the above equation has the form
where and are the mass flow rates of the cooling medium and fuel, kg/s; sk - specific heat capacity of the cooling medium, J / (kg * K); - increase in the temperature of the cooling medium, K.
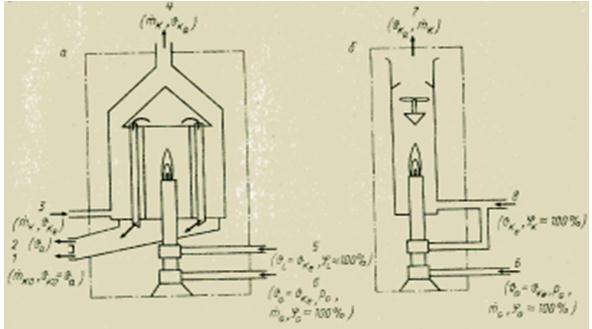
Figure Device of wet (a) and dry (b) calorimeters for gaseous fuel.
The temperature rise is typically 5-15 K. Due to the large thermal mass, heat transfer calorimeters have a very long time constant, which can be up to several minutes. Therefore, they are less suitable for use in a closed control loop as a sensor than dry (mixing) calorimeters, whose time constant is only a few seconds. On the other hand, the achievable accuracy of heat exchange calorimeters is comparatively high. Their error does not exceed ±0.25-1%, so they can also be used for laboratory work and for verification. Dry calorimeters (mixing) have an error of ±1 to ±2% of the upper limit of the measuring range.
Design versions of calorimeters from various manufacturers differ primarily in auxiliary and safety devices, sensing elements and computational circuits that provide error compensation. Thus, in heat exchange calorimeters, the ratio of gas and cooling medium flow rates is maintained in various ways (see the calorimeter equation above), due to which the higher calorific value H0 directly depends only on temperature increase.
In dry calorimeters, the temperature rise is measured either directly using electrical contact thermometers, or indirectly using a dilatometric sensor - an expanding tube located in the exhaust gas stream. In the ADOS calorimeter, the thermal elongation of the dilatometer tube corresponds directly to the heat of combustion and can be converted into any signal using a linkage and a length gauge. In the Reinecke calorimeter, the extension of the rod is used as a measuring signal in a control circuit that controls the flow of cooling air in such a way that the increase in its temperature remains almost constant. In this case, the control loop is purely proportional, but some residual deviation is inevitable in it. In this case, the consumption of cooling air or the elongation of the dilatometric tube (rod) are a measure of the determined calorific value. A prerequisite for obtaining sufficient accuracy in all dry calorimeters is good mixing of cooling air and combustion products.
HEAT FLOW MEASUREMENTS
Heat as a form of energy is transferred in three ways: through a solid body (thermal conduction), liquid or gaseous media (convection) and without the participation of matter (radiation). In technology, all three components are almost always involved in the transfer of heat; however, in many cases it is possible to obtain results of acceptable accuracy by measuring only one component.
Measurement heat flow with thermal conductivity
Heat transfer through heat-conducting walls is of great importance in many areas of technology (heat exchangers of all kinds, thermal insulation, etc.). At the same time, it is not so much the current control of production quantities that is of interest, but the results of single measurements used to assess the load, verify the fulfillment of guaranteed indicators and efficiency.
According to the laws of stationary heat conduction, the heat flux is determined by the following formulas (J/s):
Since the thermal conductivity of the wall [J/(m*s*K)] and its geometrical dimensions are known, the measurement of the heat flux is reduced to measuring the temperature difference. However, this technique requires a very accurate determination of surface temperatures. The errors associated with changes in heat transfer conditions when installing temperature sensitive elements on surfaces can be quite large. Therefore, for more accurate measurements, the methods below are recommended, in which both thermal conductivity and heat transfer are used simultaneously.
Measurement of heat flows in heat transfer (heat transfer combined with heat conduction)
For the flat wall mentioned in the previous section, the following heat transfer law (J/s) is valid:
![]() ,
,
where in the heat transfer coefficient k 1J/(m2*s*K)], along with the heat transfer coefficient [J/Dm*s*K)], the heat transfer coefficients and [J/(m2*s*K)] of both sides of the wall are also taken into account.
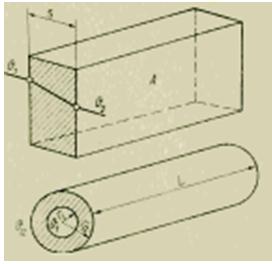
On a flat wall through which the measured heat flux passes, a small thin plate is placed, the surface temperature of which is determined by built-in thin-film thermocouples. The advantage of measuring in this way is that it does not require knowledge of the thermal properties of the wall, and the corresponding properties of the plate can be reduced to a single constant value during calibration. Such sensitive elements have a size of approximately 30x30x0.5 mm; the measurement range covers heat fluxes from 10 to 100,000 W/m2; the error is 2-5%.
Figure The principle of operation of the heat flux meter.
With the improvement of this measurement method, rubber mats are used instead of a superimposed plate. By gluing them to non-planar surfaces or wrapping them around a curved surface, it is possible to determine heat transfer from a surface of a relatively large area, for example, from a pipe, vessel, etc. Thermocouples are built into both surfaces of the mat so that their hot and cold junctions are located exactly one against another (Fig. 6). And in this case, the heat flux density in accordance with the calibration is proportional to the temperature difference. However, the applied mats somewhat disrupt the initial heat transfer, which becomes noticeable with accurate measurements. Therefore, this measurement method is mainly used to determine the thermodynamic constants of a substance, when the violation of the heat flow does not affect the measurement result.
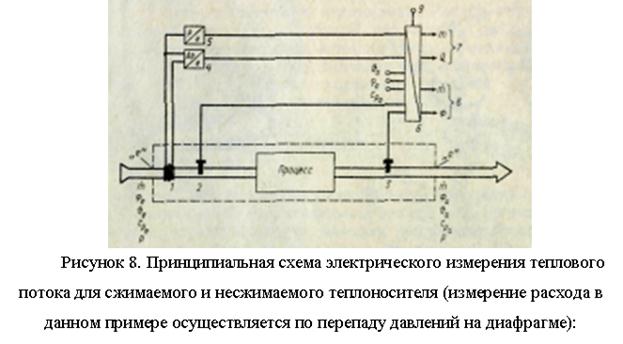
Measurement of heat flows in current environments.
A significant part of the thermal energy is transferred by liquid or gaseous media (water, steam, etc.) moving in a closed pipeline network. However, compared to the transmission of electrical energy by wire, the distance over which thermal energy can be transmitted is limited. For thermotechnical studies of all types of heating and refrigeration systems, it is necessary to measure the release and consumption of heat.
The heat flux F (J/s), transmitted by the flow of the medium - heat carrier (kg/s) through the control section with area A (m2) in a certain zone, for which the heat balance is compiled (in the process zone, Fig. 7), is equal to
The amount of heat released during the time interval t2 - t1 is determined as an integral (J):
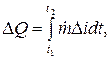
where is the difference in heat content (enthalpies, J/kg) of the coolant at the inlet (index e) and at the outlet (index a) of the heat balance zone.
Since, in the general case, the value of enthalpy is of interest only in comparison with a certain level, for example, with enthalpy at ambient temperature, all measurements of heat fluxes are essentially difference measurements.
The individual enthalpies included in the general equation can be expressed in terms of the corresponding temperatures and specific heat capacities;
Thus, the measurement of heat flow is directly reduced to the measurement of temperatures and mass flow rates. In many cases, not mass, but volume flow of the coolant is measured; in this case, the result obtained will differ only by the value of the coolant density р. The specific heat capacities, ci, are themselves functions of temperature. However, due to the narrow range of measurement of many instruments, they can usually be considered constant values without much loss of accuracy. The specific heat capacity must be known. For liquids, the heat flow equation is even more simplified, since their specific heat capacities do not depend on pressure:
![]() , J/s.
, J/s.
In all equations of this kind, it is necessary to take into account the signs of the quantities depending on whether heat is supplied or removed, whether the process is endothermic or exothermic, whether cooling or heating occurs.
