Accounting for thermal energy for "Dummies". What is thermal energy
Various building technologies and materials have their own advantages and disadvantages. So, for example, a house built of classic brick is associated with reliability for many. But what if we consider it in terms of energy efficiency? In this case, the brick will not occupy a leading position.
In order to solve the problem of thermal efficiency of buildings, various types and qualities of heaters began to be used. Starting from heat-insulating foam, which can be simply applied to certain sections of the wall of an existing house, ending with full-fledged energy-efficient wall modules. Obviously, attempts to insulate an existing house will bring some results, but will not be effective enough, including from a financial point of view. Therefore, cheap solutions appeared in the form of panels, initially equipped with insulation. These are either sandwich panels, which are foamed insulation (polystyrene) glued between DSP boards, or fibrous insulation (for example, mineral wool) embedded in the frame of a wooden wall.

More recently, the idea of using a wall panel has been refined. As a result, energy-efficient houses began to be built from full-fledged hermetic wall modules. Insulation with a record low thermal conductivity is grown inside the modules directly at the factory.

The advantage of using wall modules as part of an energy efficient building unit is their ability to best block the transfer of heat energy from the outside to the inside and vice versa. To learn to distinguish Construction Materials according to them thermophysical properties, as well as to understand why energy-efficient wall modules do their job better than sandwich panels, we will analyze all possible mechanisms for heat distribution.
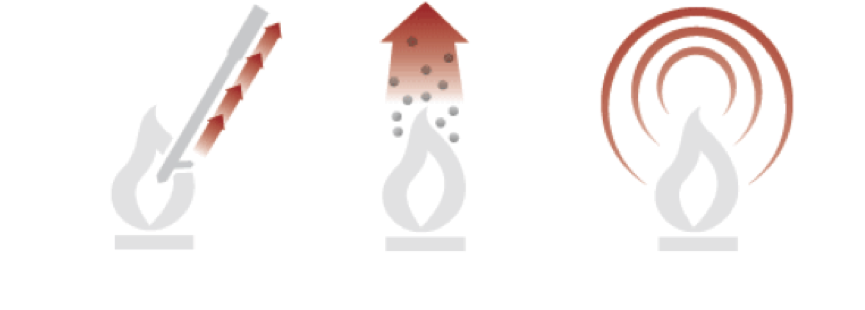
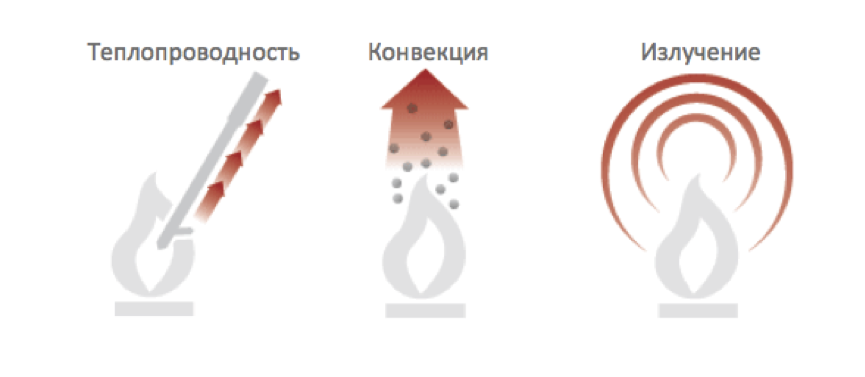
Thermal energy can be transferred through only three mechanisms: convection, heat conduction and thermal radiation.
Thermal convection occurs when hot molecules move from one place to another. The tendency of hot air to rise is the engine of natural thermal convection. Thermal conductivity is the transfer of thermal energy from one molecule to another. Each molecule may not change its position in space, but the energy will nevertheless be transferred. A hot (higher energy) molecule can transfer some of its energy to a neighboring molecule if the latter is less heated (has less energy). Roughly speaking, the denser the material, the more molecules are in contact with each other, which means more opportunities for thermal conductivity. thermal radiation(or radiation energy) is a form of electromagnetic radiation closely related to visible light. infrared electromagnetic radiation, but it propagates in exactly the same way as visible light propagates: through a vacuum, through the atmosphere, through water, and through some solids, including those that are opaque to visible light. Thus, the Sun matures the Earth through 150 million kilometers of vacuum, where there is neither the process of confection nor heat conduction. At temperatures above absolute zero (-273 C), any matter radiates some energy. These three mechanisms often work together. For example, air in a furnace is heated by conduction and radiation, diffuses through a building by convection, and heats colder objects by conduction and radiation.
Now let's look at wall panels and modules.
Inside the wall modules and panels there is a heater, which by its nature is a foamed light substance. Two conclusions follow from this. "Foamed" means few molecules in contact - low thermal conductivity, “light” means it is good reflector for thermal radiation. Due to reflection, the radiation energy is not accumulated, stored or transmitted. But the “sandwich” panel is not airtight by its design, due to which water and air seep through the panel, which means there is no blocking of the convection process. Thus, heat is dissipated by convection. But water and air cannot pass through a completely sealed wall module, which is why reduces the possibility of convection. The more airtight the module, the less the significance of the above processes.
This means that the heat from the sun stays outside the building when you try to cool the room in the summer. In winter, all the heat accumulated in the house remains inside, and does not go outside.
What is thermal energy?
Energy is the ability of a body to do work. The following types of it are distinguished: electrical, mechanical, gravitational, nuclear, chemical, electromagnetic, thermal and others.
The first is the energy of the electrons moving along the chain. Often it is used to obtain mechanical with the help of electric motors.
The second is manifested in the movement, the interaction of individual particles and bodies. deformations during tension, bending, twisting and compression of elastic bodies.
Chemical energy results from between substances. It can be released in the form of heat (for example, during combustion), as well as be converted into electrical energy (in batteries and
Electromagnetic is manifested as a result of the movement of magnetic and electric fields in the form of infrared and radio waves, etc. Nuclear is contained in radioactive substances and is released as a result of the fission of heavy nuclei or the synthesis of lungs. Gravitational - energy, which is due to the gravity of massive bodies (gravity).
Thermal energy arises in connection with the chaotic movement of molecules, atoms and other particles. It can be released as a result of mechanical action (friction), chemical or nuclear (nuclear fission). Most of the heat energy comes from combustion. various kinds fuel. It is used for heating, evaporation, heating and other technological processes.
Thermal energy is a form of energy resulting from mechanical vibrations structural elements of any substance. The parameter that allows you to determine the possibility of using it as an energy source is the energy potential. It can be expressed in kilowatt (thermal) hours or in joules.
Sources of thermal energy are divided into:
- primary. Substances possess the energy potential due to natural processes. Such sources include oceans, seas, fossil fuels, etc. Primary sources are divided into inexhaustible, renewable and non-renewable. The former include thermal waters and substances that can be used to obtain thermonuclear energy, etc. The second includes the energy of the sun, wind, water resources. Still others include gas, oil, peat, coal, etc.;
- secondary. These are substances whose energy potential directly depends on the activities of people. For example, these are heated ventilation emissions, municipal waste, hot waste heat carriers from industrial production (steam, water, gas), etc.
Thermal energy is currently produced by burning fossil fuels. The main sources are crude oil, coal, which provides 90% of the total energy consumption. However, the use of nuclear energy is increasing day by day.
Renewable sources are almost never used. This is due to the complexity of the technology for converting them into thermal energy, as well as the low energy potential of some of them.
Thermal energy arises as a result of the interaction of infrared photons with external electrons. The latter absorb photons and move to orbits far from the nucleus. Thus, the volume of the substance increases. Thermal energy is transferred through infrared photons. In particular, photons, when molecules and atoms collide with each other, jump from the zone of increased concentration of thermal energy carriers to those zones where it is lowered.
Thermal energy can be expressed in the formula: ΔQ = c.m.ΔT. C - stands for specific heat matter, m is the mass of the body, and ΔT is the temperature difference.
The heat measurement system two centuries ago was based on the idea that thermal energy is stored, does not disappear anywhere, but only moves from one place to another. We still use the following rules:
To measure the amount of heat, let's make it heat water and multiply the mass of water by the temperature increment. If the mass is taken in kg, and the difference A (temperatures) is in degrees Celsius, then their product will be heat in Cal, or kcal.
At transfer of thermal energy some other substance, then first the mass must be multiplied by the temperature increase, as for water, and then the result must be multiplied by the "specific heat" of the substance.
To measure the thermal energy released by a certain amount of fuel, a special device is needed to burn the sample and transfer the resulting heat without noticeable loss to water. Almost all types of fuel were subjected to similar tests. The weighed sample, as a rule, together with compressed oxygen, was placed in a thick metal bomb, which was immersed in a vessel with water. Then, the sample was burned with electricity and the temperature rise of the water was measured. Together with the water, the bomb with all its contents also heated up; this had to be taken into account.
Thermal energy and molecules
Any successful attempt to transfer energy to a gas heats it up, increasing the pressure (volume). AT kinetic theory we associated this with an increase in the kinetic energy of randomly moving molecules. The thermal energy of a gas is simply kinetic energy on a molecular scale. The same can be said for both liquid and solids with the only caveat that it is necessary to take into account the kinetic energy of rotation of molecules and the energy of their vibrations.
Imagine a bullet that hits an obstacle with great speed and, due to friction, gets stuck in it. In this case, the kinetic energy of the bullet is transferred to the molecules of the surrounding air and wood, giving them additional motion. Huge kinetic energy disappears, and thermal energy appears instead. If we assume that heat is a "socialized" kinetic energy, then wealth, consisting in a huge amount of ordered kinetic energy, is distributed among all randomly moving molecules - "worthy" and "unworthy". When a lead bullet strikes a wall, most of its rich store of kinetic energy is converted into vibrational energy of individual lead atoms and the wall; the energy of a trained army degenerates into a disorderly crowd.
In any discussion of issues related to the use of energy, it is necessary to distinguish between thermal energy (the energy of chaotic motion) and the energy of ordered motion, known in technology as free energy. So, the kinetic energy of a flying bullet is the energy of an ordered movement - it is all contained in the pool. We call it free energy because it can be turned into potential energy in its entirety; To do this, you just need to shoot vertically up! The deformation energy is also ordered, and we also call it free energy, because the spring can spend it on lifting the load. Almost all chemical energy is free, as is electrical energy and the energy of high-temperature radiation. Any of these forms of energy allows you to use all the energy. Chaotic thermal energy has one significant drawback. No matter what tricks we go for, only a part of the heat can turn into mechanical energy.
This is due to the fact that even in the best of conceivable machines to convert heat into mechanical energy, some of the heat is transferred to the refrigerator. Otherwise, the machine will not be able to repeat the work cycle. We are not able to completely order the random movement of molecules, turning its energy into free. Some chaos will always remain. A thought experiment with an ideal heat engine says that the maximum proportion of heat that can be used is (T1-T2) / T1, where T1 is the absolute temperature of the “heater”, or boiler, and T2 is the absolute temperature of the refrigerator of the machine (about the meaning of absolute temperature see chapter 27). Yes, steam under high pressure with a temperature of 500 ° K (227 ° C), turning into water with a temperature of 300 ° K (27 ° C), can give an efficiency of no more than (500-300) / 500, or 40% Such a steam engine should throw away, in addition to real losses, 60% of their heat.
From this it becomes quite obvious that thermal energy and thermal engines are the bottleneck in modern energy. All machines are engaged in continuous thermal energy production, and its ejection into environment. Moreover, if it is quite possible to solve the problems of efficient conversion into electrical energy by improving semiconductor and nanotechnologies, then the problem of low efficiency of a heat engine cannot be solved.
The maximum efficiency is (T1-T2)/T1, or 1-(T2/T1). So the higher T1 (or the lower T2), the closer the efficiency is to unity. To reduce costs, power plants are trying to do with the highest possible temperature T1 of the heater, or boiler. Serious limitations arise from the oil starting to burn and the metal starting to melt. The temperature T2, with a constant supply of heat, cannot be made lower than the ambient temperature for a long time. In practice, we have no way to use chemical or atomic energy directly. We must first convert it into thermal energy, and only after that we cannot avoid large thermal losses.
Paradoxical as it may seem, but the same reasoning based on thought experiments says that when another need arises - to get heat from free energy, i.e. when we want to heat an apartment with electricity, we can achieve high efficiency (k.p. d.).
Using free energy, with the help of a small machine, we can “pump” heat energy from a cold street into a warm room. In essence, such a heat pump for thermal energy consumption a refrigerator turned inside out, the freezer compartment of which is placed outside the room, can serve.
By using sunlight, coal, or water to do useful work, like powering electric lamps, driving a lathe, or pumping water to the top of a hill, etc., we again and again come to thermal energy as an almost inevitable by-product (due to friction) and the most likely final product. When the light of the lamp is absorbed by the walls, the machine cuts the metal or the water flows back into the ocean, the energy originally received from the fuel, in the end, is completely converted into heat. And if we were dealing with heat at the beginning, then at the final stage there will be a lower temperature. It is practically unsuitable for further use. You can, of course, come up with another end - let the light radiate into interstellar space, the machine to twist the spring, and leave the water on top of the hill, but, as a rule, the end product is still thermal energy. (All the energy from the combustion of gasoline in all the cars in the world over the past year, eventually passed into heating the air and the earth - this is how it turns out).
Simply about the complex – Thermal energy
- Gallery of images, pictures, photos.
- Determining the amount of thermal energy, energy loss - basics, opportunities, prospects, development.
- Interesting facts, useful information.
- Green news - Determining the amount of thermal energy, energy loss.
- Links to materials and sources - Thermal energy.
I won't give a dictionary definition here. thermal energy . I will try to explain everything on the fingers. The article is not for experts.
Think what's different hot water from cold, what affects the temperature of the water?
It differs in the amount of heat contained in it. This warmth, or in other words thermal energy, cannot be seen or touched, it can only be felt. Any water with a temperature greater than 0°C contains some amount of heat. The higher the temperature of water (steam or condensate), the more heat it contains.
Heat is measured in Calories, in Joules, in MWh (Megawatts per hour), not in degrees °C.
Since the tariffs are approved in hryvnia per Gigacalorie, we will take Gcal as a unit of measurement.
Thus, hot water consists of the water itself and the thermal energy or heat (Gcal) contained in it. Water seems to be saturated with gigacalories. The more Gcal in the water, the hotter it is. Sometimes hot water is called a heat carrier, i.e. brings warmth.
In heating systems, the coolant (hot water) enters the heating system at one temperature and exits at another. That is, he came with one amount of warmth, and left with another. The coolant gives off some of the heat to the environment through heating radiators. For this part, which did not return to the system, and which is measured in Gcal, someone has to pay
In case of hot water supply (or a rush in the heating system), we consume all the water and, accordingly, all 100% Gcal in it, we do not return anything back to the system.
Thus, when installing metering units in an apartment building or a private house, we will pay directly for the heat consumed (Gcal) by our premises. If there is no metering device, we will be charged the amount for the heat we consumed. by tariff". Moreover, this "at the rate" can be several times higher than the amount of heat actually consumed by us. That is why today, more than ever, the question of installing thermal energy metering units arises.
What is thermal energy accounting.
A thermal energy metering unit is a complex of devices, which is why it is called a node.
Technically it looks like this. The following are cut into the pipelines of heating networks (into the supply, into the return, into the DHW network):
- flow meters - measure the amount of coolant passed;
- temperature sensors - measure the temperature of the coolant;
- and (not always) pressure sensors - measure pressure in pipelines.
The devices need to be supplied with some kind of voltage, autonomous or mains, depending on the type of device.
These devices must be inserted as close as possible to the border of the balance sheet (BP) and operational responsibility (EO), i.e. to the place where your networks begin. The heat supply contract must have an appropriate act or annex.
If the devices do not crash at the border of the BP and EO, then the heat supply company calculates heat losses in the section of heat networks from the BP boundary to the place of installation of the recording devices for each pipeline, taking into account the method of laying (underground / ground), the diameter of the network and the presence of thermal insulation of pipelines.
Payment for heat losses is charged in addition to the readings of the heat metering unit by the balance method. In the invoice for payment, they are usually allocated as a separate line. In some heat supply companies, heat losses are not taken into account, they are calculated according to the readings of the heat meter.
From measuring instruments the wires send signals to a heat recorder, or a heat meter, or a heat meter, as you like. The heat recorder records the data in its memory and stores in its archive a period determined by the manufacturer.
For example, hourly readings can be stored for the last 15 days, daily readings for the last 45 days, monthly readings for the last 12 months.
Based on these data, the heat recorder mathematically calculates Gcal, for which we pay.
However, the installation of a thermal energy metering unit does not lead to savings!
If you install a heat metering unit and at the same time assume that now happiness has come - this is a complete delusion! To save money, it is necessary that the heat supply company begin to charge less, in fact, “according to the meter”. For this it is necessary take data from the meter and transfer it to the heating network ! This is what will save you money!
