Which substances have poor thermal conductivity. II international competition of research and creative works of students "start in science
1 Morozovsk, Branch of the University Cossack Cadet Boarding School State University technologies and management named after K.G. Razumovsky (First Cossack University)", 8/1 platoon
Mosina O.V. (Morozovsk, Branch of the University Cossack Cadet Corps-Boarding FSBEI HE "Moscow State University of Technology and Management named after K.G. Razumovsky (First Cossack University)")
Peryshkin A.V. Physics grade 8. – M.: Bustard, 2012.
Bludov M.I. Conversations on physics part 1. - M .: Education, 1984.
URL: http://class-fizika.narod.ru/8_3.htm.
URL: http://en.wikipedia.org/wiki/ %D0 %BE %D0 %B4 %D0 %BD %D0 %BE %D1 %81 %D1 %82 %D1 %8C.
The project is designed in accordance with the standard of medium general education in physics. When writing this project, the study of thermal phenomena, their application in everyday life and technology was considered. In addition to theoretical material, much attention is paid to research work - these are experiments that answer the questions "In what ways can the internal energy of the body be changed", "Is the thermal conductivity the same various substances”, “Why do jets of warm air or liquid rise upwards”, “Why do bodies with a dark surface heat up more”; search and processing of information, photos.
Time of work on the project: 1 - 1.5 months.
Project goals:
- practical implementation of schoolchildren's knowledge about thermal phenomena;
- formation of skills of independent research activity;
- development of cognitive interests;
- development of logical and technical thinking;
- development of abilities for independent acquisition of new knowledge in physics in accordance with vital needs and interests;
Main part
Theoretical part
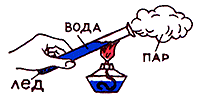
In life, we really daily meet with thermal phenomena. However, we do not always think that these phenomena can be explained if you know physics well. In physics lessons, we got acquainted with ways to change internal energy: heat transfer and doing work on the body or the body itself.
When two bodies with different temperatures come into contact, energy is transferred from a body with more high temperature to a body with a lower temperature. This process will continue until the temperatures of the bodies are equal (thermal equilibrium is reached). In this case, no mechanical work is done. The process of changing internal energy without doing work on the body or the body itself is called heat transfer or heat transfer. In heat transfer, energy is always transferred from a hotter body to a cooler one. The reverse process never occurs spontaneously (on its own); heat exchange is irreversible. Heat transfer determines or accompanies many processes in nature: the evolution of stars and planets, meteorological processes on the surface of the Earth, etc. Types of heat transfer: thermal conductivity, convection, radiation.
Thermal conductivity is the phenomenon of energy transfer from more heated parts of the body to less heated ones as a result of thermal movement and interaction of the particles that make up the body.
Metals have the highest thermal conductivity - they have hundreds of times more than water. The exceptions are mercury and lead, but even here the thermal conductivity is ten times greater than that of water.
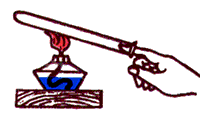
When lowering a metal needle into a glass of hot water, very soon the end of the needle became hot too. Consequently, internal energy, like any kind of energy, can be transferred from one body to another. Internal energy can also be transferred from one part of the body to another. So, for example, if one end of a nail is heated in a flame, then its other end, which is in the hand, will gradually heat up and burn the hand.
Practical part
Let us study this phenomenon by doing a series of experiments with solids, liquids and gases.
Have taken various items: one aluminum spoon, another wooden spoon, a third plastic spoon, a fourth stainless steel spoon, and a fifth silver spoon. We attached paper clips to each spoon with drops of honey. They put spoons into a glass of hot water so that handles with paper clips stick out of it in different directions. The spoons will heat up and as they heat up the honey will melt and the paper clips will fall off.
Of course, the spoons should be the same in shape and size. Where heating occurs faster, that metal conducts heat better, is more thermally conductive. For this experiment, I took a glass of boiling water and four types of spoons: aluminum, silver, plastic and stainless. I lowered them one by one into a glass and timed the time: in how many minutes it would heat up. That's what I did:
Conclusion: spoons made of wood and plastic take longer to heat up than spoons made of metal, which means that metals have good thermal conductivity.
Let's bring the end of a wooden stick into the fire. It will ignite. The other end of the stick, which is outside, will be cold. This means that the tree has poor thermal conductivity.
We bring the end of a thin glass rod to the flame of an alcohol lamp. After a while, it will heat up, while the other end will remain cold. Therefore, glass has poor thermal conductivity

If we heat the end of a metal rod in a flame, then very soon the entire rod will become very hot. We can no longer hold it in our hands.
This means that metals conduct heat well, that is, they have a high thermal conductivity. A rod is fixed horizontally on a tripod. On the rod, at regular intervals, metal studs are vertically fixed with wax.


A candle is brought to the edge of the rod. Since the edge of the rod heats up, the rod gradually warms up. When heat reaches the point where the studs are attached to the stem, the stearin melts and the stud falls off. We see that in this experiment there is no transfer of matter, respectively, thermal conductivity is observed.
![]()

Different metals have different thermal conductivity. In the physics room there is a device with which we can make sure that different metals have different thermal conductivity. However, at home, we were able to verify this with the help of a home-made device.
display device different thermal conductivity solids.
We have made a device for displaying different thermal conductivity of solids. To do this, we used an empty aluminum foil jar, two rubber rings (homemade), three pieces of wire made of aluminum, copper and iron, a tile, hot water, 3 figurines of men with arms raised, cut out of paper.
The order of manufacture of the device:
1. bend the wire in the form of the letter "G";
2. strengthen them from the outside of the jar with rubber rings;
3. hang paper men from the horizontal parts of the wire segments (using molten paraffin or plasticine).
Checking the operation of the device. Pour into a jar hot water(if necessary, heat a jar of water on an electric stove) and observe which figure will fall first, second, third.
Results. The first figure to fall is fixed on a copper wire, the second - on aluminum, the third - on steel.
Conclusion. Various solids have different thermal conductivity.
The thermal conductivity of different substances is different.
Consider now the thermal conductivity of liquids. Take a test tube with water and begin to heat its upper part. The water at the surface will soon boil, and at the bottom of the tube during this time it will only heat up. This means that liquids have low thermal conductivity.

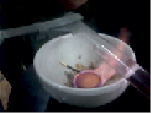


We investigate the thermal conductivity of gases. We put a dry test tube on the finger and heat it upside down in the flame of an alcohol lamp. The finger will not feel warm for a long time. This is due to the fact that the distance between gas molecules is even greater than that of liquids and solids. Therefore, the thermal conductivity of gases is even less.

Wool, hair, bird feathers, paper, snow and others have poor thermal conductivity. porous bodies.
![]()
This is due to the fact that air is contained between the fibers of these substances. Air is a poor heat conductor.


So green grass is preserved under the snow, winter crops are preserved from freezing.
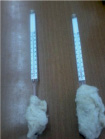
He fluffed up a small ball of cotton wool and wrapped it around a thermometer ball.
Now he held the thermometer for some time at a certain distance from the flame and noticed how the temperature rose. Then the same ball of cotton squeezed and wrapped tightly around the bulb of the thermometer and again brought it to the lamp. In the second case, mercury will rise much faster.
This means that compressed cotton wool conducts heat much better!
If there is a need to protect the body from cooling or heating, then substances with low thermal conductivity are used. So, for pots, pans, handles are made of plastic or wood.


Houses are built from logs or bricks, which have poor thermal conductivity, which means they are protected from cooling.

Vacuum (space freed from air) has the lowest thermal conductivity. This is explained by the fact that thermal conductivity is the transfer of energy from one part of the body to another, which occurs during the interaction of molecules or other particles. In a space where there are no particles, heat conduction cannot take place.
Conclusion
Different substances have different thermal conductivity.
Solids (metals) have high thermal conductivity, liquids have less thermal conductivity, and gases have poor thermal conductivity.
We can use the thermal conductivity of various substances in everyday life, technology and nature.
The phenomenon of thermal conductivity is inherent in all substances, regardless of the state of aggregation they are in.
Now, without difficulty, I can answer and explain from a physical point of view to the questions:
1. Why do birds fluff their feathers in cold weather?
(There is air between the feathers, and air is a poor conductor of heat.)
2. Why do woolen clothes keep the cold better than synthetic ones?
(There is air between the hairs, which does not conduct heat well).
3. Why do cats sleep curled up in a ball when the weather is cold in winter? (Curled into a ball, they reduce the surface area that gives off heat).
4. Why are the handles of soldering irons, irons, pans, pots made of wood or plastic? (Wood and plastic have poor thermal conductivity, so when we heat metal objects, we will not burn our hands when holding a wooden or plastic handle).
5. Why are bushes of heat-loving plants and bushes covered with sawdust for the winter?
(Sawdust is a poor conductor of heat. Therefore, plants are covered with sawdust so that they do not freeze).
6. Which boots protect against frost better: tight or spacious?
(Spacious, since air does not conduct heat well, it is another layer in the boot that retains heat).
Bibliographic link
Belyaevsky I.A. RESEARCH OF THERMAL CONDUCTIVITY OF VARIOUS SUBSTANCES // International School Scientific Bulletin. - 2017. - No. 1. - P. 72-76;URL: https://school-herald.ru/ru/article/view?id=143 (date of access: 07.11.2017).
Heat transfer is one of the ways to change the internal energy of a body (or system of bodies), while the internal energy of one body is transferred to the internal energy of another body without performing mechanical work.
There are 3 types of heat transfer:
Heat exchange between two media occurs through a solid wall separating them or through the interface between them.
Heat can only transfer from a body with a higher temperature to a body with a lower temperature.
Heat exchange always proceeds in such a way that a decrease in the internal energy of some bodies is always accompanied by the same increase in the internal energy of other bodies participating in heat exchange.
This is a special case of the law of conservation of energy.
INTERESTING
Partridges, ducks and other birds do not freeze in winter because the temperature of their paws can differ from body temperature by more than 30 degrees. The low temperature of the paws greatly reduces heat transfer. These are defensive forces organism!
Thermal conductivity is the transfer of energy from more heated parts of the body to less heated ones due to thermal movement and interaction of microparticles (atoms, molecules, ions, etc.), which leads to equalization of body temperature.
Not accompanied by transfer of substance!
This type of transfer of internal energy is characteristic of both solids and liquids and gases.
The thermal conductivity of various substances is different.
Metals have the highest thermal conductivity,

and different metals have different thermal conductivity.
Liquids have less thermal conductivity than solids, and gases less than liquids.
When heating the upper end of a test tube closed with a finger with air inside, you can not be afraid to burn your finger, because. the thermal conductivity of gases is very low.
It is interesting that it would be possible to bring your hand almost close to the flame, for example, of a gas burner (temperature more than 1000 degrees) and not burn it if ...
What if?

Gas is generally a very poor conductor of heat, so just a small layer of air between the hand and the flame would suffice. But!
But there is such a phenomenon as convection in gases, therefore, near the flame, the hand burns strongly.
LOOK AT THE BOOKSHELF
Do you know that...

Great difficulties for the builders of buildings are delivered by the subsidence of the foundation, especially in regions with permafrost. Houses often crack due to the thawing of the soil beneath them. The foundation transfers some amount of heat to the soil. Therefore, buildings began to be built on piles. In this case, heat is transferred only by thermal conductivity from the foundation to the pile and further from the pile to the ground. What should piles be made of? It turns out that piles made of durable solid material must be filled with kerosene inside. In summer, the pile conducts heat from top to bottom poorly, because. liquid has low thermal conductivity. In winter, due to the convection of the liquid inside the pile, on the contrary, it will contribute to additional cooling of the soil.
This is not a fairy tale, not fantasy!
Such a project is really designed and tested!
Italian scientists have invented a shirt that allows you to maintain a constant body temperature. Scientists promise that it will not be hot in summer and cold in winter, because it is made of special materials. Similar materials are already used in space flights.
In the old machine guns "Maxim" heating water protected the weapon from melting.
In the kitchen, when lifting dishes filled with hot liquid, in order not to burn yourself, you can use only a dry rag. The thermal conductivity of air is much less than that of water! And the fabric structure is very loose, and all the gaps between the fibers are filled with air in a dry rag, and water in a wet one. Look, don't get burned!
Fire in the sieve

The phenomenon described below demonstrates the property of metals to conduct heat well.
If you make a mesh of wire, providing a good metal connection at the places where the wire crosses, and place it above a gas burner, then you can ignite the gas above the mesh when the valve is turned on, while it will not burn under the mesh. And if you ignite the gas under the grid, then the fire “will not leak” up through the grid!
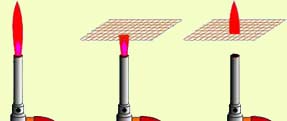
In those days, when there were no electric miner's light bulbs, they used the Davy lamp.
It was a candle "planted" in a metal cage. And even if the shaft was filled with flammable gases, the Davy lamp was safe and did not cause an explosion - the flame did not go beyond the lamp, thanks to the metal mesh.
Thermal conductivity is a type of heat transfer in which there is a direct transfer of energy from particles (molecules, atoms) of a more heated part of the body to particles of its less heated part.
Consider a series of experiments with heating a solid, liquid and gas.
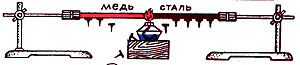
We fix a thick copper wire in a tripod, and attach a few carnations to the wire with wax or plasticine. When the free end of the wire is heated in the flame of an alcohol lamp, the wax melts, and the cloves gradually fall off the wire. And first those that are closer to the flame fall off, then all the rest in turn. This is explained as follows. First, the speed of movement of those metal particles that are closer to the flame increases. The temperature of the wire in this place rises. When these particles interact with neighboring ones, the speed of the latter also increases, as a result of which the temperature of the next part of the wire rises. Then the speed of movement of the following particles increases, and so on, until the entire wire warms up.
It should be remembered that during heat conduction, the substance itself does not move along the body, only energy is transferred.
Consider now the thermal conductivity of liquids. Take a test tube with water. We put a piece of ice in it and begin to heat the top of the test tube. The surface water will soon boil. The ice at the bottom of the test tube will hardly melt during this time. This means that liquids have low thermal conductivity, with the exception of mercury and liquid metals.
This is due to the fact that in liquids the molecules are located at greater distances from each other than in liquids. solids.
We investigate the thermal conductivity of gases. We put a dry test tube on a finger and heat the bottom in the flame of an alcohol lamp. The finger does not feel warm for a long time.
This is due to the fact that the distance between gas molecules is even greater than that of liquids and solids. Therefore, the thermal conductivity of gases is even less.
So, the thermal conductivity of various substances is different.
Metals, especially silver and copper, have the highest thermal conductivity. If the thermal conductivity of various substances is compared with the thermal conductivity of copper, it turns out that for iron it is about 5 times less, for water - 658 times, for porous brick - 848 times, for freshly fallen snow - almost 4000 times, for cotton wool , sawdust and sheep wool - almost 10,000 times, and for air it is about 20,000 times less. Hair, feathers, paper, cork and other porous bodies also have poor thermal conductivity. This is due to the fact that air is contained between the fibers of these substances. Vacuum (space freed from air) has the lowest thermal conductivity. This is explained by the fact that thermal conductivity is the transfer of energy from one part of the body to another, which occurs during the interaction of molecules or other particles. In a space where there are no particles, heat conduction cannot take place.
If there is a need to protect the body from cooling or heating, then substances with low thermal conductivity are used. So, the handles for pots and pans are made of plastic. Houses are built from logs or bricks, which have poor thermal conductivity, which means they protect the premises from cooling. The device of a thermos, or Dewar vessel, which was invented in 1892 by the English scientist James Dewar, is based on the use of vacuum as a heat-insulating "material".
1. Introduction.
The project was developed in accordance with the standard of secondary general education in physics. When writing this project, the study of thermal phenomena, their application in everyday life and technology was considered. In addition to theoretical material, much attention is paid to research work - these are experiments that answer the questions “In what ways can the internal energy of a body be changed”, “Is the thermal conductivity of various substances the same”, “Why do jets of warm air or liquid rise up”, “Why bodies with dark the surface heats up more "; search and processing of information, photographs. Time of work on the project: 1 - 1.5 months. Project goals: * practical implementation of schoolchildren's knowledge about thermal phenomena; * formation of skills for independent research; * development of cognitive interests; * development of logical and technical thinking * development of abilities for independent acquisition of new knowledge in physics in accordance with vital needs and interests;
2. The main part.
2.1. Theoretical part
In life, we really daily meet with thermal phenomena. However, we do not always think that these phenomena can be explained if you know physics well. In physics lessons, we got acquainted with ways to change internal energy: heat transfer and doing work on the body or the body itself. When two bodies with different temperatures come into contact, energy is transferred from a body with a higher temperature to a body with a lower temperature. This process will continue until the temperatures of the bodies are equal (thermal equilibrium is reached). In this case, no mechanical work is done. The process of changing internal energy without doing work on the body or the body itself is called heat transfer or heat transfer. In heat transfer, energy is always transferred from a hotter body to a cooler one. The reverse process never occurs spontaneously (by itself), i.e., heat transfer is irreversible. Heat transfer determines or accompanies many processes in nature: the evolution of stars and planets, meteorological processes on the surface of the Earth, etc. Types of heat transfer: thermal conductivity, convection, radiation.
thermal conductivity called the phenomenon of energy transfer from more heated parts of the body to less heated as a result of thermal motion and the interaction of the particles that make up the body.
Metals have the highest thermal conductivity - they have hundreds of times more than water. The exceptions are mercury and lead, but even here the thermal conductivity is ten times greater than that of water.
When lowering a metal needle into a glass of hot water, very soon the end of the needle became hot too. Consequently, internal energy, like any kind of energy, can be transferred from one body to another. Internal energy can also be transferred from one part of the body to another. So, for example, if one end of a nail is heated in a flame, then its other end, which is in the hand, will gradually heat up and burn the hand.
2.2. Practical part.
Let us study this phenomenon by doing a series of experiments with solids, liquids and gases.
Experience #1
They took various objects: one aluminum spoon, another wooden one, the third one made of plastic, the fourth one made of stainless steel, and the fifth one was silver. We attached paper clips to each spoon with drops of honey. They put spoons into a glass of hot water so that handles with paper clips stick out of it in different directions. The spoons will heat up and as they heat up the honey will melt and the paper clips will fall off.
Of course, the spoons should be the same in shape and size. Where heating occurs faster, that metal conducts heat better, is more thermally conductive. For this experiment, I took a glass of boiling water and four types of spoons: aluminum, silver, plastic and stainless. I lowered them one by one into a glass and timed the time: in how many minutes it would heat up. That's what I did:
Conclusion: spoons made of wood and plastic take longer to heat up than spoons made of metal, which means that metals have good thermal conductivity.
Experience #2
Let's bring the end of a wooden stick into the fire. It will ignite. The other end of the stick, which is outside, will be cold. This means that the tree has poor thermal conductivity.
We bring the end of a thin glass rod to the flame of an alcohol lamp. After a while, it will heat up, while the other end will remain cold. Therefore, glass also has poor thermal conductivity.
If we heat the end of a metal rod in a flame, then very soon the entire rod will become very hot. We can no longer hold it in our hands.
This means that metals conduct heat well, that is, they have a high thermal conductivity. On the shta-ti-ve go-ri-zon-tal-but fortified-lyon ster-zhen. On the rod, through one-on-one space, ver-ti-kal-but fasten-le-na with the help of wax metal carnations.
To the edge of the rod, they put a candle under it. Since the edge of the rod is on the gre-va-et-sya, then in a degree-pen-but the ster-zhen pro-gre-va-et-sya. When the heat reaches the place where the carnations with the rod are fastened, the ste-a-rin melts, and the carnation falls. We see that in this experiment there is no pe-re-but-sa-substance, so-respectively-but, ob-observe-yes-there-is-warm-lo-water- ness.
Experience #3
Different metals have different thermal conductivity. In the physics room there is a device with which we can make sure that different metals have different thermal conductivity. However, at home, we were able to verify this with the help of a home-made device.
An instrument for displaying the various thermal conductivities of solids.
We have made a device for displaying different thermal conductivity of solids. To do this, we used an empty aluminum foil jar, two rubber rings (homemade), three pieces of wire made of aluminum, copper and iron, a tile, hot water, 3 figurines of men with arms raised, cut out of paper.
The order of manufacture of the device:
bend the wire in the form of the letter "G";
strengthen them from the outside of the can with rubber rings;
hang paper men from the horizontal parts of the wire segments (using molten paraffin or plasticine).
Checking the operation of the device. Pour hot water into a jar (if necessary, heat a jar of water on an electric stove) and observe which figure will fall first, second, third.
Results. The first figure to fall is fixed on a copper wire, the second - on aluminum, the third - on steel.
Conclusion. Different solids have different thermal conductivity.
The thermal conductivity of different substances is different.
Experience No. 4
Consider now the thermal conductivity of liquids. Take a test tube with water and begin to heat its upper part. The water at the surface will soon boil, and at the bottom of the tube during this time it will only heat up. This means that liquids have low thermal conductivity.
Experience No. 5
We investigate the thermal conductivity of gases. We put a dry test tube on the finger and heat it upside down in the flame of an alcohol lamp. The finger will not feel warm for a long time. This is due to the fact that the distance between gas molecules is even greater than that of liquids and solids. Therefore, the thermal conductivity of gases is even less.
Wool, hair, bird feathers, paper, snow and other porous bodies have poor thermal conductivity.
This is due to the fact that air is contained between the fibers of these substances. Air is a poor heat conductor.
So green grass is preserved under the snow, winter crops are preserved from freezing.
Experience No. 6
He fluffed up a small ball of cotton wool and wrapped it around the thermometer ball. Now he held the thermometer for a while at a certain distance from the flame and noticed how the temperature rose. Then the same ball of cotton squeezed and wrapped tightly around the bulb of the thermometer and again brought it to the lamp. In the second case, mercury will rise much faster. This means that compressed cotton wool conducts heat much better!
Vacuum (space freed from air) has the lowest thermal conductivity. This is explained by the fact that thermal conductivity is the transfer of energy from one part of the body to another, which occurs during the interaction of molecules or other particles. In a space where there are no particles, heat conduction cannot take place.
3. Conclusion.
Different substances have different thermal conductivity.
Solids (metals) have high thermal conductivity, liquids have less thermal conductivity, and gases have poor thermal conductivity.
We can use the thermal conductivity of various substances in everyday life, technology and nature.
The phenomenon of thermal conductivity is inherent in all substances, regardless of the state of aggregation they are in.
Now, without difficulty, I can answer and explain from a physical point of view to the questions:
1. Why do birds fluff their feathers in cold weather?
(There is air between the feathers, and air is a poor conductor of heat.)
2. Why do woolen clothes keep the cold better than synthetic ones?
(There is air between the hairs, which does not conduct heat well).
3. Why do cats sleep curled up in a ball when the weather is cold in winter? (Curled into a ball, they reduce the surface area that gives off heat).
4. Why are the handles of soldering irons, irons, pans, pots made of wood or plastic? (Wood and plastic have poor thermal conductivity, so when we heat metal objects, we will not burn our hands when holding a wooden or plastic handle).
5. Why are bushes of heat-loving plants and bushes covered with sawdust for the winter?
(Sawdust is a poor conductor of heat. Therefore, plants are covered with sawdust so that they do not freeze).
6. Which boots protect against frost better: tight or spacious?
(Spacious, since air does not conduct heat well, it is another layer in the boot that retains heat).
4. List of used literature.
Printed editions:
1.A.V. Peryshkin Physics Grade 8 -M: Bustard, 2012
2.M.I.Bludov Conversations on physics part 1 -M: Enlightenment 1984.
Internet resources:
1.http://class-fizika.narod.ru/8_3.htm
2.http://ru.wikipedia.org/wiki/%D0%A2%D0%B5%D0%BF%D0%BB%D0%BE%D0%BF%D1%80%D0%BE%D0%B2 %D0%BE%D0%B4%D0%BD%D0%BE%D1%81%D1%82%D1%8C
ROUTING
IN PHYSICS IN 8 CLASS
Maintain interest in the subject
To form the communication skills of the work of students
Build respect for classmates
Requirements of GEF LLC
(assumed by learning outcomes)
Personal
Convince in the possibility of understanding nature, in the need for the reasonable use of the achievements of science and technology for the further development of human society;
Respect for the creators of science and technology;
Attitude to physics as an element of social culture.
Metasubject
COGNITIVE
Analysis of the problem experiment;
Performing actions according to the algorithm;
Formation mental operations knowledge: comparisons, generalizations, modeling, abstraction, analysis
REGULATORY:
Acceptance of the learning goal;
Drawing up a sequence of actions to discover new knowledge;
Orientation in a decision-making situation.
COMMUNICATIVE:
The ability to reason, conduct a dialogue, listen to the teacher;
subject
Understanding physical foundations thermal conductivity of different bodies and their application;
Formation of the ability to explain the results of the experiment, in terms of knowledge on the topic
Subject: THERMAL CONDUCTIVITY
Goal: a positive attitude to work in the classroomHello guys, I hope everyone is in a good mood. Is everyone ready for the lesson? So let's start the lesson. Look out the window, what a beautiful autumn. The cold is coming soon, are we ready for it? What should you do to keep warm in winter? How to protect plants from frost? Today's lesson will help us answer these questions.
Check readiness for the lesson.
Answer questions, discuss.
IIstage. Knowledge update
Target: Review previously learned material to lead to learning new topic
List ways to change internal energy.
Name the types of heat transfer.
Work done and heat transfer.
Heat conduction, convection, radiation.
II. Motivation for activity
Solve the rebus
Thermal conductivity
Purpose: to stimulate interest in the subject
Learning new material
Purpose: to introduce the concept of thermal conductivity, the transfer process, use.
What do you think the topic of our lesson is? What questions will we consider?
1. Thermal conductivity
Demonstration of experiences. Based on them, conclusions are drawn
1. A spoon is lowered into a glass of hot water. What will happen to the spoon?
2. Why is the spoon hot?
3. As a result of what is the transfer of heat from the heated end of the spoon to the cold?
What conclusion can be drawn?
Heating a spoon in hot tea is an example of heat conduction.
Thermal conductivity - the transfer of energy from more heated parts of the body to less heated ones, as a result of thermal motion and interaction of particles.
Let us study this phenomenon by doing a series of experiments with solids, liquids and gases.
Let's do the experiments:
Let's bring the end of a wooden stick into the fire. It will ignite. The other end of the stick, which is outside, will be cold.
We bring the end of a thin glass rod to the flame of an alcohol lamp. After a while, it will heat up, while the other end will remain cold.
We heat the end of the metal rod, then soon the entire rod will heat up.
Let's fix the end copper wire in the foot of the tripod. Carnations are attached to the wire with wax. We will heat the free end of the wire with the flame of an alcohol lamp.
What are we seeing?
How does heat transfer take place?
How long will the heat transfer through the wire take?
What can be said about the speed of movement of molecules in the area located closer to the flame?
Why does the next piece of wire heat up?
Consider now the thermal conductivity of liquids. Take a test tube with water and begin to heat its upper part. What are we seeing? Do you think the bottom is hot. Feel. What is the conclusion?Dand is small except for mercury and molten metals.
We investigate the thermal conductivity of gases. We put a dry test tube on the finger and heat it upside down in the flame of an alcohol lamp. The finger will not feel warm for a long time.
Wool, bird feathers, paper, cork and other porous substances have poor thermal conductivity. This is due to the fact that air is contained between the fibers of these substances. Vacuum has the lowest thermal conductivity
Let's write down the mainthermal conductivity features:
in solids, liquids and gases;
the substance itself is not tolerated;
leads to equalization of body temperature;
different bodies - different thermal conductivity
The topic of the lesson is thermal conductivity.
What is thermal conductivity. The process of transferring energy by heat conduction. Which bodies have good and poor thermal conductivity. Where knowledge of thermal conductivity is applied.
Students write the topic of the lesson in their notebooks.
She will warm up.
The water gave off some of the heat to the spoon and some to the surrounding air.
- As a result of the movement and interaction of particles
Conclusion:
It is clear from the example that heat can be transferred from a body that is hotter to a body that is less heated (from hot water to a cold spoon). But the energy was also transferred along the spoon itself - from its heated end to the cold one.
Write down the definition.
Conclusion. Wood has poor thermal conductivity.
Conclusion. Glass has poor thermal conductivity.
Conclusion. Metals have a high thermal conductivity.
- The carnations begin to gradually fall one by one, first those closest to the flame.
From the hot end of the wire to the cold end.
Until the entire wire is heated, that is, until the temperature in the entire wire is equalized.
Molecules move faster.- As a result of the interaction of molecules, the speed of movement of molecules in the next section also increases and the temperature of this part increases.
The water boiled at the surface.
The day is slightly warm.
Conclusion. Liquids have low thermal conductivity.
Conclusion. The thermal conductivity of gases is even lower.
The thermal conductivity of different substances is different.
Record the main features of thermal conductivity
They name which bodies have good, poor thermal conductivity. Fill in the table in your notebook
Anchoring
Purpose: to consolidate the material, to get to know where in life we apply the knowledge of thermal conductivity.
Let's remember a fairy tale
Heat Conduction Examples :
An excerpt from the fairy tale "Moroz Ivanovich"
The needlewoman began to whip the snow so that the old man could sleep softer, but meanwhile, her poor hands ossified and her fingers turned white, like those of poor people, who rinse their linen in the hole in winter: it’s cold, and the wind in the face, and the linen freezes, stake worth it, but there is nothing to do - poor people work.
Nothing, - said Moroz Ivanovich, - just rub your fingers with snow, and they will go away, you won’t get chills. I'm a kind old man; look at my curiosities. Then he lifted his snowy featherbed with a blanket, and the Needlewoman saw that green grass was breaking through under the featherbed. The needlewoman felt sorry for the poor weed.
So you say, - she said, - that you are a good old man, but why do you keep green grass under a snowy feather bed, do not let it out into the light of day?
I don’t release because it’s not time yet; The grass hasn't come into play yet. In the autumn, the peasants sowed it, and it sprouted, and if it had already stretched out, then winter would have captured it, and by the summer the grass would not have ripened. So I covered the young greenery with my snowy feather bed, and even lay down on it myself so that the snow would not be blown away by the wind; but then spring will come, the snowy feather bed will melt, the grass will begin to grow, and there, you look, the grain will look out, and the peasant will collect the grain and take it to the mill ...
Why do people plant winter crops and are not afraid that they will freeze?
Why do plant bushes cover the winter with sawdust?
What do we use in the kitchen so as not to get burned?
What are frying pans made of? Why?
Why do woolen clothes keep out the cold better than synthetic ones?
Interesting facts from biology. A shaggy fur coat allows bumblebees to collect nectar and pollen even in the Arctic. Under such clothes, the body of a bumblebee, with increased muscle work, heats up to 40 0 . And the further north a bumblebee lives, the larger and shaggy it is. Why does a fur coat save a bumblebee from freezing?
As soon as the cold sets in, the bees crowd on the combs with honey and form a dense ball. Clinging to each other, they maintain a temperature of about 12 0 C. Thus, in winter, the bees warm themselves. But they need ventilation, because otherwise all the moisture exhaled by the bees settles inside the hive in the form of frost. Why do bees manage to keep themselves warm in winter?
Which brick - solid or porous - provides better thermal insulation of the building? Justify the answer.
At the same temperature of granite and brick, brick feels warmer to the touch than granite. Which of these building materials has the best thermal insulation property?
Scissors and a pencil lying on the table have the same temperature. Why do scissors feel colder to the touch?
The considered examples will help us to draw a conclusion and fill in the table.
They listen to the text, name the bodies with good and poor thermal conductivity.
Snow is a porous, loose substance, it contains air. Therefore, snow has poor thermal conductivity and well protects the earth, winter crops, fruit trees from freezing.
Kitchen potholders are made of a material that has poor thermal conductivity.
Frying pans and pans are made of substances with good thermal conductivity (metals) and are used to quickly heat bodies or parts.
Handles of teapots, pans are made of materials with poor thermal conductivity. All this protects hands from burns when touching hot objects.
Sawdust is a poor conductor of heat. Plants are covered with sawdust so that they do not freeze.
There is air between the hairs, which does not conduct heat well.
The coat of a bumblebee does not conduct heat well, since there is air between the villi, whose thermal conductivity is low.
Air remains between the bees, which conducts heat poorly and prevents freezing.
Let's have a mini research work. Let's find out if the expression is true: THE FUR COAT WARMS ?!
How will we do the work?
To do this, we need a thermometer, and a piece of fur. Let's measure the temperature of the room, then put the thermometer in a flap for a while
Draw a conclusion
Primary knowledge test
Purpose: to check at what level the material is understood?
heat capacity
calorific value
thermal conductivity
Conduction is the transfer of matter from one body to another
Heat conduction does NOT transfer matter from one body to another.
The concept of thermal conductivity does not exist
Wood
Glass
Copper
What is the name of the phenomenon of transfer of internal energy from one part of the body to another or from one body to another when they are in direct contact?
Choose the correct statement.
Which of the following substances has highest thermal conductivity?
Answer in the student's card.
Lesson summary
Recall the questions at the beginning of the lesson. Are we ready for winter? What phenomenon are we seeing today? What is this phenomenon?
Homework. P 4, (all), prepare a report "Thermal conductivity in nature, everyday life and technology." (optional)
Thank you for your work in class.
Answer questions
