What is the thermal conductivity of porous bodies and why
Heat transfer in nature is carried out with the help of heat conduction, convection and radiation (radiation absorption and emission).
The mechanism of heat conduction is actually explained in the previous paragraph. Let's take another example. When the end of a metal rod is heated, its molecules begin to move faster, i.e., the internal energy of this end increases. Since the molecules move more slowly at the other end of the rod, inside the rod, with the help of the chaotic movement of atoms and electrons, internal energy is transferred from the hot to the cold end. The transfer of internal energy from one part of a substance to another, due to the chaotic movement of molecules and other particles of a substance, is called thermal conductivity.
Among various kinds metals have the best thermal conductivity. This is due to the fact that they contain free electrons. We also note that the thermal conductivity of a substance in the solid state is greater than in the liquid state, and in the liquid state it is greater than in the gaseous state.
Consider the essence of convection. To show the poor thermal conductivity of water, usually a vessel of water is heated from above. At the same time, the water can boil at the top, but remain cold at the bottom. However, if the vessel is heated from below, then the water is heated evenly throughout the volume. This is explained by the fact that water expands when heated and its density decreases. If the heated water is at the bottom, then the upper, denser layers of water descend under the action of gravity and displace the warm water up. This mixing of water will continue until all the water boils. The heat transfer that occurs when unevenly heated layers of liquid or gas are mixed under the action of gravity is called convection. It is easy to see that convection is absent in a spacecraft in a state of weightlessness.(Consider why the freezer in refrigerators is reinforced at the top instead of the bottom.)
It may seem that convection cannot be considered heat transfer, since it is associated with the work of gravity. However, during convection, an increase in the internal energy of a liquid or gas occurs only due to the heat supplied from outside, and the effect of gravity is reduced only to accelerating the uniform heating of the liquid or gas. The action of gravity during convection does not give an additional contribution to the internal energy of a liquid or gas. Therefore, convection is referred to as heat transfer.
Heat exchange between the Sun and the Earth is carried out by means of electromagnetic radiation. Electromagnetic radiation is created by the movement of electric charges and increases sharply with increasing temperature. The radiation of a body, which is determined only by its temperature, is called thermal radiation.
The process of radiation occurs due to the internal energy of the body . When radiation is absorbed by some other body, the internal energy of the body increases due to the energy of the absorbed radiation.Thus, by means of radiation, energy is transferred from more heated bodies to less heated ones. This type of heat transfer occurs even in the absence of matter between the bodies.
In the previous paragraph, we found out that when a metal needle was lowered into a glass of hot water, very soon the end of the spoke also became hot. Consequently, internal energy, like any kind of energy, can be transferred from one body to another. Internal energy can also be transferred from one part of the body to another. So, for example, if one end of a nail is heated in a flame, then its other end, which is in the hand, will gradually heat up and burn the hand.
The phenomenon of transfer of internal energy from one part of the body to another or from one body to another when they are in direct contact is called heat conduction.
Let us study this phenomenon by doing a series of experiments with solids, liquids and gases.
Let's put the end of a wooden stick into the fire. It will ignite. The other end of the stick, which is outside, will be cold. So the tree has poor thermal conductivity .
We bring the end of a thin glass rod to the flame of the spirit lamp. After a while, it will heat up, while the other end will remain cold. Consequently, glass also has poor thermal conductivity.

If we heat the end of a metal rod in a flame, then very soon the entire rod will become very hot. We can no longer hold it in our hands.
This means that metals conduct heat well, that is, they have great thermal conductivity. The highest thermal conductivity have silver and copper.
Consider the transfer of heat from one part of a solid body to another in the following experiment.
We fix one end of the thick copper wire in a tripod. Attach a few carnations to the wire with wax. When the free end of the wire is heated in the flame of an alcohol lamp, the wax will melt. The carnations will start to fall off gradually (Fig. 5). First, those that are closer to the flame will disappear, then all the rest in turn.
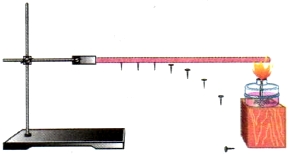
Rice. 5. Transfer of heat from one part of a solid body to another
Let's find out how energy is transferred along the wire. The speed of the oscillatory movement of metal particles increases in that part of the wire that is closer to the flame. Since particles constantly interact with each other, the speed of movement of neighboring particles increases. The temperature of the next piece of wire begins to rise, and so on.
It should be remembered that during heat conduction there is no transfer of matter from one end of the body to the other.
Consider now the thermal conductivity of liquids. Take a test tube with water and begin to heat its upper part. The water at the surface will soon boil, and at the bottom of the test tube, during this time, it will only heat up (Fig. 6). This means that liquids have low thermal conductivity, with the exception of mercury and molten metals.
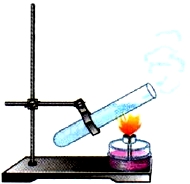
Rice. 6. Thermal conductivity of liquid
This is due to the fact that in liquids the molecules are located at greater distances from each other than in solids.
We investigate the thermal conductivity of gases. We put a dry test tube on a finger and heat it in the flame of an alcohol lamp with the bottom up (Fig. 7). The finger will not feel warm for a long time.
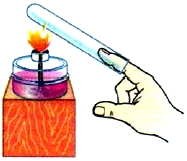
Rice. 7. Gas thermal conductivity
This is due to the fact that the distance between gas molecules is even greater than that of liquids and solids. Therefore, the thermal conductivity of gases is even less.
So, thermal conductivity at various substances different.
The experience shown in Figure 8 shows that the thermal conductivity of different metals is not the same.
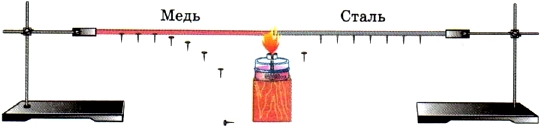
Rice. 8. Thermal conductivity of different metals
Wool, hair, bird feathers, paper, cork and other porous bodies have poor thermal conductivity. This is due to the fact that air is contained between the fibers of these substances. Vacuum (space freed from air) has the lowest thermal conductivity. This is explained by the fact that thermal conductivity is the transfer of energy from one part of the body to another, which occurs during the interaction of molecules or other particles. In a space where there are no particles, heat conduction cannot take place.
If there is a need to protect the body from cooling or heating, then substances with low thermal conductivity are used. So, for pots, pans, handles are made of plastic. Houses are built from logs or bricks, which have poor thermal conductivity, which means that they protect the premises from cooling.
Questions
- How is energy transferred through a metal wire?
- Explain the experience (see Fig. 8) showing that the thermal conductivity of copper is greater than the thermal conductivity of steel.
- Which substances have the highest and lowest thermal conductivity? Where are they used?
- Why do fur, down, feathers on the body of animals and birds, as well as human clothing protect from the cold?
Exercise 3
- Why does deep loose snow protect winter crops from freezing?
- It is estimated that the thermal conductivity of pine boards is 3.7 times greater than that of pine sawdust. How to explain such a difference?
- Why doesn't water freeze under a thick layer of ice?
- Why is the expression "fur coat warm" incorrect?
Exercise
Take a cup of hot water and dip a metal and wooden spoon into the water at the same time. Which spoon will heat up faster? How is heat exchanged between water and spoons? How does the internal energy of water and spoons change?
Synopsis of a lesson in physics in grade 8: "Types of heat transfer".
Lesson Objectives:
To introduce students to the types of heat transfer.
To form the ability to explain the thermal conductivity of bodies in terms of the structure of matter; be able to analyze video information; explain observed phenomena.
Lesson type: combined lesson.
Demos:
1. Heat transfer along a metal rod.
2. Video demonstration of an experiment comparing the thermal conductivity of silver, copper and iron.
3. Rotation of a paper pinwheel over a switched on lamp or tile.
4. Video demonstration of the occurrence of convection currents when water is heated with potassium permanganate.
5. Video demonstration on the radiation of bodies with a dark and light surface.
DURING THE CLASSES
II. Reporting the topic and objectives of the lesson
In the previous lesson, you learned that internal energy can be changed by doing work or by transferring heat. Today in the lesson we will look at how the change in internal energy occurs by heat transfer.
Try to explain the meaning of the word "heat transfer" (the word "heat transfer" implies the transfer of thermal energy). There are three ways to transfer heat, but I will not name them, you yourself will name them when you solve the puzzles.
Answers: conduction, convection, radiation.
Let's get acquainted with each type of heat transfer separately, and let the words of M. Faraday become the motto of our lesson: "Observe, study, work."
III. Learning new material
1. Thermal conductivity
Answer the questions:
1. What happens if we put a cold spoon into hot tea? (It will warm up after a while).
2. Why does a cold spoon get hot? (The tea gave up part of its heat to the spoon and part to the surrounding air).
Conclusion: It is clear from the example that heat can be transferred from a body that is more heated to a body that is less heated (from hot water to a cold spoon). But the energy was also transferred along the spoon itself - from its heated end to the cold one.
3. As a result of what is the transfer of heat from the heated end of the spoon to the cold? (As a result of the movement and interaction of particles)
Heating a spoon in hot tea is an example of heat conduction.
Thermal conductivity- the transfer of energy from more heated parts of the body to less heated ones, as a result of thermal motion and interaction of particles.
Let's experiment:
Fix the end of the copper wire in the foot of the tripod. Carnations are attached to the wire with wax. We will heat the free end of the wire of candles or on the flame of an alcohol lamp. 
Questions:
1. What are we observing? (Carnations begin to gradually fall off one by one, first those that are closer to the flame).
2. How does heat transfer take place? (From the hot end of the wire to the cold end).
3. How long will the heat transfer through the wire take? (Until the entire wire is heated, that is, until the temperature in the entire wire is equalized)
4. What can be said about the speed of movement of molecules in the area located closer to the flame? (Molecules move faster)
5. Why is the next piece of wire heating up? (As a result of the interaction of molecules, the speed of movement of molecules in the next section also increases and the temperature of this part increases)
6. Does the distance between molecules affect the rate of heat transfer? (The smaller the distance between the molecules, the faster the heat transfer occurs)
7. Remember the arrangement of molecules in solids Ah, liquids and gases. In which bodies the process of energy transfer will occur faster? (Faster in metals, then in liquids and gases).
Watch the demonstration of the experiment and be prepared to answer my questions. 
Questions:
1. On which plate does heat spread faster, and on which one more slowly?
2. Make a conclusion about the thermal conductivity of these metals. (Better thermal conductivity for silver and copper, somewhat worse for iron)
Note that there is no body transfer during heat transfer in this case.
Wool, hair, bird feathers, paper, cork and other porous bodies have poor thermal conductivity. This is due to the fact that air is contained between the fibers of these substances. Vacuum (space freed from air) has the lowest thermal conductivity.
Let's write down the main thermal conductivity features:
in solids, liquids and gases;
the substance itself is not tolerated;
leads to equalization of body temperature;
different bodies - different thermal conductivity
Heat conduction examples:
1. Snow is a porous, loose substance, it contains air. Therefore, snow has poor thermal conductivity and well protects the earth, winter crops, fruit trees from freezing.
2. Kitchen potholders are made of a material that has poor thermal conductivity. Handles of teapots, pans are made of materials with poor thermal conductivity. All this protects hands from burns when touching hot objects.
3. Substances with good thermal conductivity (metals) are used to quickly heat bodies or parts.
2. Convection
Guess the riddles:
1) Look under the window -
There is an accordion stretched out
But the harmonica does not play -
It warms our apartment ... (battery)
2) Our fat Fedora
eats soon.
But when you're full
From Fedora - warmth ... (oven)
Batteries, stoves, heating radiators are used by a person to heat residential premises, or rather, to heat the air in them. This happens due to convection - the next type of heat transfer.
Convection is the transfer of energy by jets of liquid or gas.
Let's try to explain how convection occurs in residential premises.
The air, in contact with the battery, heats up from it, while it expands, its density becomes less than the density of cold air. Warm air, being lighter, rises up under the action of the Archimedes force, and heavy cold air sinks down.
Then again: colder air reaches the battery, heats up, expands, becomes lighter and rises up under the action of the Archimedean force, etc.
Due to this movement, the air in the room warms up.
A paper pinwheel placed over a switched on lamp begins to rotate.
Try to explain how it happens? (Cold air when heated at the lamp becomes warm and rises, while the spinner rotates).
The liquid is heated in the same way. Look at the experiment on observing convection currents when water is heated (using potassium permanganate).
Note that, unlike thermal conduction, convection involves the transfer of matter, and convection does not occur in solids.
There are two types of convection: natural and forced.
Heating a liquid in a pot or air in a room are examples natural convection. For its occurrence, substances must be heated from below or cooled from above. Why exactly? If we heat from above, then where will the heated layers of water move, and where will the cold ones? (Answer: nowhere, since the heated layers are already at the top, and the cold layers will remain below)
Forced convection is observed if the liquid is stirred with a spoon, pump or fan.
Convection features:
occurs in liquids and gases, is impossible in solids and vacuum;
the substance itself is transferred;
substances must be heated from below.
Convection examples:
1) cold and warm sea and ocean currents,
2) in the atmosphere, vertical air movements lead to the formation of clouds;
3) cooling or heating of liquids and gases in various technical devices, for example, in refrigerators, etc., water cooling of engines is provided
internal combustion.
3. Radiation
Everyone knows that The sun is the main source of heat on Earth. The earth is located at a distance of 150 million km from it. How is heat transferred from the Sun to the Earth?
Between the Earth and the Sun, outside of our atmosphere, all space is a vacuum. And we know that heat conduction and convection cannot occur in a vacuum.
How is heat transferred? Here another type of heat transfer is carried out - radiation.
Radiation is heat transfer in which energy is transferred by electromagnetic rays.
It differs from heat conduction and convection in that heat in this case can be transferred through a vacuum.
Watch a video about radiation.
All bodies radiate energy: the human body, the stove, the electric lamp.
The higher the body temperature, the more thermal radiation.
Bodies not only radiate energy, but also absorb it.
Moreover, dark surfaces absorb and radiate energy better than bodies with a light surface.
Features of radiation:
occurs in any substance;
the higher the body temperature, the more intense the radiation;
takes place in a vacuum;
dark bodies absorb radiation better than light bodies and radiate better.
Examples of the use of body radiation:
surfaces of rockets, airships, balloons, satellites, airplanes, are painted with silver paint so that they do not heat up by the Sun. If, on the contrary, it is necessary to use solar energy, then parts of the devices are painted in a dark color.
People wear dark clothes in winter (black, blue, cinnamon), they are warmer, and light in summer (beige, white colors). Dirty snow melts faster in sunny weather than clean snow, because bodies with a dark surface absorb solar radiation better and heat up faster.
IV. Consolidation of the acquired knowledge on examples of tasks
Game "Try, explain".
Before you is a playing field with six tasks, you can choose any. After completing all the tasks, you will open wise saying and the one who very often pronounces it from TV screens.
1. Which house is warmer in winter if the thickness of the walls is the same? warmer in wooden house, since wood contains 70% air, and brick 20%. Air is a poor conductor of heat. Recently, "porous" bricks have been used in construction to reduce thermal conductivity.
2. How is energy transferred from the heat source to the boy? To the boy sitting by the stove, the energy is mainly transferred by heat conduction.
3. How is energy transferred from the heat source to the boy?
To a boy lying on the sand, energy from the sun is transferred by radiation, and from the sand by heat conduction.
4. In which of these wagons are perishable products transported? Why? Perishable products are transported in wagons painted in White color, since such a car is heated to a lesser extent by the sun's rays.
5. Why don't waterfowl and other animals freeze in winter?
Fur, wool, down have poor thermal conductivity (the presence of air between the fibers), which allows the body of the animal to store the energy produced by the body and protect itself from cooling.
6. Why are window frames made double?
Between the frames contains air, which has poor thermal conductivity and protects against heat loss.
“The world is more interesting than we think”, Alexander Pushnoy, Galileo program.
V. Summary of the lesson
What types of heat transfer are we familiar with?
– Determine which type of heat transfer plays a major role in the following situations:
a) heating water in a kettle (convection);
b) a person warms himself by a fire (radiation);
c) heating of the table surface from the included table lamp (radiation);
d) heating a metal cylinder immersed in boiling water (thermal conduction).
VI. Homework
§ 4, 5, 6, Ex. 1 (3), Ex. 2(1), Ex. 3(1) - in writing.
VII. Reflection
At the end of the lesson, we invite students to discuss the lesson: what they liked, what they would like to change, evaluate their participation in the lesson.
People also have different thermal conductivity, some warm like fluff, while others take heat like iron.
Yuri Serezhkin
The word "also" in the above statement shows that the concept of "thermal conductivity" is applied to people only conditionally. Although…
Did you know: a fur coat does not heat, it only retains the heat that the human body produces.
This means that the human body has the ability to conduct heat in a literal, and not just figurative sense. This is all poetry, in fact, we will compare heaters in terms of thermal conductivity.
You know better, because you yourself typed in the search engine "thermal conductivity of heaters." What exactly did you want to know? And if without jokes, then it is important to know about this concept, because different materials behave very differently when used. An important, although not a key point in the choice is precisely the ability of the material to conduct thermal energy. If you choose the wrong heat-insulating material, it simply will not perform its function, namely, to keep the heat in the room.
Step 2: Theory concept
From school course Physicists will most likely remember that there are three types of heat transfer:
- Convection;
- Radiation;
- Thermal conductivity.
So thermal conductivity is a type of heat transfer or movement of thermal energy. It has to do with the internal structure of the bodies. One molecule transfers energy to another. Now would you like a little test?
Which type of substance transmits (transfers) the most energy?
- Solid bodies?
- Liquids?
- Gases?
That's right, the crystal lattice of solids transfers energy most of all. Their molecules are closer to each other and therefore can interact more effectively. Gases have the lowest thermal conductivity. Their molecules are at the greatest distance from each other.
Step 3: What can be a heater
We continue our conversation about the thermal conductivity of heaters. All bodies that are nearby tend to equalize the temperature among themselves. A house or apartment, as an object, seeks to equalize the temperature with the street. Are all building materials capable of being insulators? No. For example, concrete allows the heat flow from your house to the street too quickly, so the heating equipment will not have time to maintain the desired temperature in the room. The thermal conductivity coefficient for insulation is calculated by the formula:
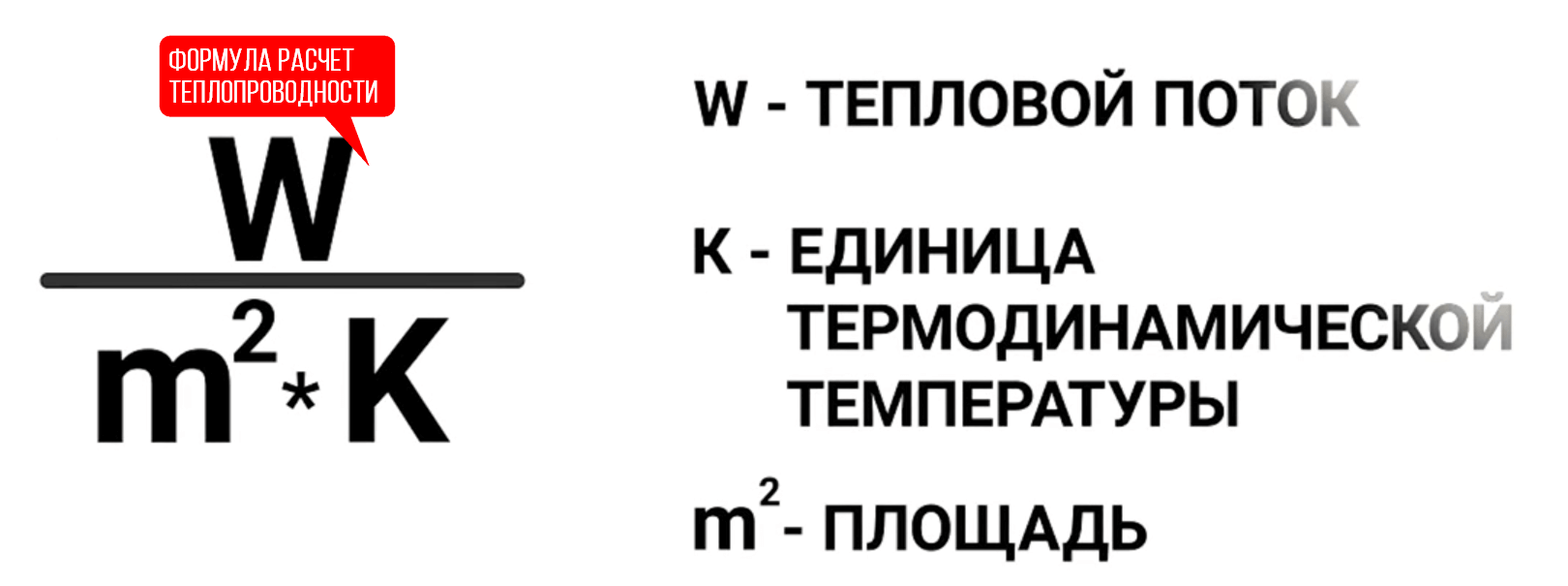
Where W is our heat flux, and m2 is the area of \u200b\u200binsulation with a temperature difference of one Kelvin (It is equal to one degree Celsius). For our concrete, this coefficient is 1.5. This means that conditionally, one square meter of concrete with a temperature difference of one degree Celsius is able to pass 1.5 watts of thermal energy per second. But, there are materials with a coefficient of 0.023. It is clear that such materials are much better suited for the role of heaters. Does thickness matter, you ask? Plays. But, here you still can not forget about the heat transfer coefficient. To achieve the same results, you will need a concrete wall 3.2 m thick or a sheet of foam plastic 0.1 m thick. It is clear that although concrete can technically be a heater, it is not economically feasible. That's why:
Insulation can be called a material that conducts the least amount of thermal energy through itself, preventing it from leaving the room and at the same time costing as little as possible.

The best heat insulator is air. Therefore, the task of any insulation is to create a fixed air gap without convection (movement) of air inside it. That is why, for example, foam plastic is 98% air. The most common insulating materials are:
- Styrofoam;
- extruded polystyrene foam;
- mineral wool;
- Penofol;
- Penoizol;
- Foam glass;
- Polyurethane foam (PPU);
- Ecowool (cellulose);
The thermal insulation properties of all the materials listed above lie close to these limits. It is also worth considering: the higher the density of the material, the more it conducts energy through itself. Remember from theory? The closer the molecules are, the more efficiently heat is conducted.
Step 4: Compare. Table of thermal conductivity of heaters
The table shows a comparison of heaters in terms of thermal conductivity declared by manufacturers and corresponding to GOSTs:
Comparative table of thermal conductivity building materials, which are not considered to be heaters:
The heat transfer rate only indicates the rate of heat transfer from one molecule to another. For real life, this indicator is not so important. But you can’t do without a thermal calculation of the wall. Heat transfer resistance is the reciprocal of thermal conductivity. We are talking about the ability of the material (insulation) to retain heat flow. To calculate the resistance to heat transfer, you need to divide the thickness by the coefficient of thermal conductivity. The example below shows the calculation of the thermal resistance of a wall made of a 180 mm thick beam.

As you can see, the thermal resistance of such a wall will be 1.5. Enough? It depends on the region. The example shows the calculation for Krasnoyarsk. For this region, the required coefficient of resistance of enclosing structures is set at 3.62. The answer is clear. Even for Kyiv, which is much further south, this figure is 2.04.
Thermal resistance is the reciprocal of thermal conductivity.
This means that abilities wooden house resisting heat loss is not enough. Warming is necessary, and already, with what material - calculate according to the formula.
Step 5: Mounting Rules
It is worth saying that all the above indicators are given for DRY materials. If the material gets wet, it will lose its properties by at least half, or even turn into a “rag”. Therefore, it is necessary to protect thermal insulation. Styrofoam is most often insulated under a wet facade, in which the insulation is protected by a layer of plaster. A waterproofing membrane is applied to the mineral wool to prevent moisture from entering.

Another point that deserves attention is wind protection. Heaters have different porosity. For example, let's compare expanded polystyrene boards and mineral wool. If the first one looks solid, the second one clearly shows pores or fibers. Therefore, if you are installing fibrous thermal insulation, such as mineral wool or ecowool, on a wind-blown fence, be sure to take care of the wind protection. Otherwise, the good thermal performance of the insulation will not be useful.
conclusions
So, we discussed that the thermal conductivity of heaters is their ability to transfer thermal energy. The heat insulator must not release the heat generated by the heating system of the house. The primary task of any material is to keep air inside. It is the gas that has the lowest thermal conductivity. It is also necessary to calculate the thermal resistance of the wall in order to find out the correct coefficient of thermal insulation of the building. If you have any questions about this topic, please leave them in the comments.
Three interesting facts about thermal insulation
- The snow serves as a heat insulator for the bear in the den.
- Clothing is also a heat insulator. We are not very comfortable when our body tries to equalize temperature with temperature. environment, which can be -30 degrees, instead of the usual 36.6.
- The blanket is a thermal insulator. It does not allow the heat of the human body to escape.
Bonus
As a bonus for the curious who have read to the end an interesting experiment with thermal conductivity:
