Which body has the highest thermal conductivity? VI. Homework. III. Learning new material
In the previous paragraph, we found out that when lowering a metal needle into a glass with hot water very soon the end of the spoke became hot too. Consequently, internal energy, like any kind of energy, can be transferred from one body to another. Internal energy can also be transferred from one part of the body to another. So, for example, if one end of a nail is heated in a flame, then its other end, which is in the hand, will gradually heat up and burn the hand.
Diagram showing the transfer of thermal energy through conduction. Heat is an interesting form of energy. Not only does it keep us alive, make us comfortable, and help us prepare our food, but understanding its properties is the key to many areas of scientific research. For example, knowing how heat is transferred and the extent to which different materials can exchange thermal energy drives everything from building heaters to understanding seasonal changes to send ships into space.
Heat can only be transferred in three ways: conduction, convection and radiation. Of these, conduction is perhaps the most common and occurs regularly in nature. In short, it is transmission through physical contact. This happens when you press your hand on a windowpane, when you place a pot of water on an active element, and when you place iron on a fire.
The phenomenon of transfer of internal energy from one part of the body to another or from one body to another when they are in direct contact is called heat conduction.
Let us study this phenomenon by doing a series of experiments with solids, liquids and gases.
Let's put the end of a wooden stick into the fire. It will ignite. The other end of the stick, which is outside, will be cold. So the tree has poor thermal conductivity.
This transfer occurs at the molecular level - from one body to another - when thermal energy is absorbed by the surface and causes the surface molecules to move faster. In the process, they collide with their neighbors and transfer energy to them, a process that continues as long as heat is added.
IV. Consolidation of the acquired knowledge on examples of tasks
The process of thermal conduction depends on four main factors: the cross section of those involved, their path lengths, and the properties of these materials. The temperature gradient is physical quantity, which describes in which direction and at what rate the temperature changes in a particular place. Temperature always flows from the hottest to the coldest source, due to the fact that cold is nothing but the absence of thermal energy. This transfer between bodies continues until the temperature difference decays and a state known as thermal equilibrium occurs.
We bring the end of a thin glass rod to the flame of the spirit lamp. After a while, it will heat up, while the other end will remain cold. Therefore, glass has poor thermal conductivity.

If we heat the end of a metal rod in a flame, then very soon the entire rod will become very hot. We can no longer hold it in our hands.
An important factor is also the cross section and path length. The larger the size of the material associated with the transfer, the more heat is needed to heat it. In addition, the greater the surface area exposed to open air, the more likely it is to. So shorter objects with smaller cross sections are best remedy loss minimization.
Thermal conduction occurs through any material represented here by a rectangular rod. The rate at which transfer occurs depends in part on the thickness of the material. Last, but certainly not least, physical properties materials. Basically, when it comes to conductive heat, not all substances are created equal. Metals and stone are considered good conductors because they can transfer heat quickly, while materials such as wood, paper, air, and cloth are poor heat conductors.
This means that metals conduct heat well, that is, they have great thermal conductivity. Silver and copper have the highest thermal conductivity.
Consider the transfer of heat from one part of a solid body to another in the following experiment.
We fix one end of the thick copper wire in a tripod. Attach a few carnations to the wire with wax. When the free end of the wire is heated in the flame of an alcohol lamp, the wax will melt. The carnations will start to fall off gradually (Fig. 5). First, those that are closer to the flame will disappear, then all the rest in turn.
These conductive properties are evaluated based on a "factor" which is measured relative to silver. In this regard, silver has a factor of 100, while other materials are ranked lower. These include copper, iron, water and wood. At the opposite end of the spectrum, there is an ideal vacuum, which is unable to conduct heat, and therefore evaluates to zero.
Materials that are poor conductors of heat are called insulators. Air, which has a conductivity coefficient of 0.006, is an exceptional insulator because it is able to be contained in an enclosed space. That's why artificial insulators use air compartments, like double-glazed windows that are used to cut heating bills. Basically, they act as buffers against heat loss.
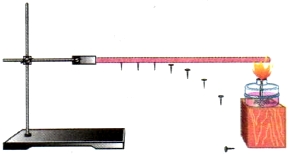
Rice. 5. Transfer of heat from one part of a solid body to another
Let's find out how energy is transferred along the wire. The speed of the oscillatory movement of metal particles increases in that part of the wire that is closer to the flame. Since particles constantly interact with each other, the speed of movement of neighboring particles increases. The temperature of the next piece of wire begins to rise, and so on.
Feathers, furs, and natural fibers are all examples of natural insulators. These are materials that keep birds, mammals and humans warm. Sea otters, for example, live in ocean waters that are often very cold, and their luxuriously thick fur keeps them warm. Other marine mammals such as sea lions, whales and penguins rely on thick layers of blubber - a very poor conductor - to prevent heat loss through the skin.
The same logic applies to insulating houses, buildings, and even spacecraft. In these cases, methods include either trapped air pockets between walls, fiberglass, or high-density foam. The spacecraft is a special case and uses insulation in the form of foam, reinforced carbon composite and silica tiles. These are all poor conductors of heat and therefore prevent heat loss in space, as well as preventing extreme temperatures caused by precipitation from entering the flight deck.
It should be remembered that during heat conduction there is no transfer of matter from one end of the body to the other.
Consider now the thermal conductivity of liquids. Take a test tube with water and begin to heat its upper part. The water at the surface will soon boil, and at the bottom of the test tube, during this time, it will only heat up (Fig. 6). This means that liquids have low thermal conductivity, with the exception of mercury and molten metals.
Conduction, as evidenced by the heating of a metal rod with a flame. The laws governing the conduction of heat are very similar to Ohm's Law, which governs electrical conduction. In this case, a good conductor is a material that allows electric current to flow through it without too much trouble. In contrast, an electrical insulator is any material whose internal electrical charges do not flow freely, and therefore it is very difficult to conduct an electric current when subjected to an electric field.
In most cases, materials that are poor conductors of heat are also poor conductors of electricity. For example, copper is a good conductor of both heat and electricity, which is why copper wires are widely used in electronics manufacturing. Gold and silver are even better, and where price is not an issue, these materials are also used in the construction of electrical circuits.
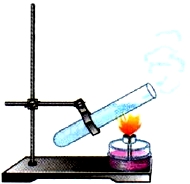
Rice. 6. Thermal conductivity of liquid
This is due to the fact that in liquids the molecules are located at greater distances from each other than in liquids. solids Oh.
We investigate the thermal conductivity of gases. We put a dry test tube on a finger and heat it in the flame of an alcohol lamp with the bottom up (Fig. 7). The finger will not feel warm for a long time.
And when someone is looking to "ground" the charge, they send it through the physical connection to the Earth, where the charge is lost. This is common in electrical circuits where exposed metal is a factor in ensuring that people who accidentally come into contact do not undergo electromutation.
Insulating materials, such as rubber on the soles of shoes, are worn to protect people from working on sensitive materials or from electrically charged power supplies. Others, such as glass, polymers, or porcelain, are commonly used on power lines and high voltage power transmitters to keep power flowing through circuits.
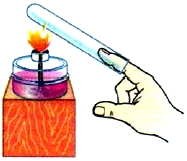
Rice. 7. Gas thermal conductivity
This is due to the fact that the distance between gas molecules is even greater than that of liquids and solids. Therefore, the thermal conductivity of gases is even less.
So, thermal conductivity at various substances different.
The experience shown in Figure 8 shows that the thermal conductivity of different metals is not the same.
In short, conduction comes down to the transfer of heat or the transfer of electric charge. Both of these occur as a result of the ability of matter to transfer energy through them. Black objects do not induce heat. Black objects absorb incoming radiation in the visible range. Similarly, white objects do not reflect heat. They diffusely reflect incoming visible radiation.
But these are colors. Whether blacks or whites are "colors" depends a lot on what you mean by color. For this question, it's better to look at black and white as shades of gray rather than colors like red and blue. What is this physics? The answer lies in the concepts of emissivity, absorptivity, reflectivity, and transmittance. Emissivity is the ability of an object to emit thermal radiation about an ideal black body.
- Absorbability is the proportion of incoming radiation absorbed by an object.
- Reflectivity is the proportion of incoming radiation reflected by an object.
- Transmission is the proportion of incoming radiation that passes through an object.
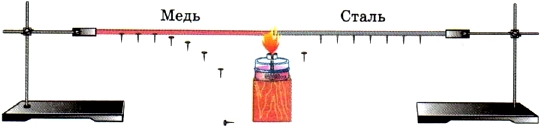
Rice. 8. Thermal conductivity of different metals
Wool, hair, bird feathers, paper, cork and others have poor thermal conductivity. porous bodies. This is due to the fact that air is contained between the fibers of these substances. Vacuum (space freed from air) has the lowest thermal conductivity. This is explained by the fact that thermal conductivity is the transfer of energy from one part of the body to another, which occurs during the interaction of molecules or other particles. In a space where there are no particles, heat conduction cannot take place.
II. Reporting the topic and objectives of the lesson
They are added to 1. Incoming light for opaque objects is either absorbed or reflected in a ratio determined by the object's absorptivity and reflectance. Reflectivity and absorptivity explain part of why black objects get hotter than white ones. A perfectly black object absorbs all incoming visible radiation, while a perfectly white object reflects all incoming visible radiation. Since there is no such thing as a perfectly black or completely white object, all objects absorb incoming visible radiation to some extent.
If there is a need to protect the body from cooling or heating, then substances with low thermal conductivity are used. So, for pots, pans, handles are made of plastic. Houses are built from logs or bricks, which have poor thermal conductivity, which means that they protect the premises from cooling.
Questions
- How is energy transferred through a metal wire?
- Explain the experience (see Fig. 8) showing that the thermal conductivity of copper is greater than the thermal conductivity of steel.
- Which substances have the highest and lowest thermal conductivity? Where are they used?
- Why do fur, down, feathers on the body of animals and birds, as well as human clothing protect from the cold?
Exercise 3
- Why does deep loose snow protect winter crops from freezing?
- It is estimated that the thermal conductivity of pine boards is 3.7 times greater than that of pine sawdust. How to explain such a difference?
- Why doesn't water freeze under a thick layer of ice?
- Why is the expression "fur coat warm" incorrect?
Exercise
Take a cup of hot water and dip a metal and wooden spoon into the water at the same time. Which spoon will heat up faster? How is heat exchanged between water and spoons? How does the internal energy of water and spoons change?
However, black objects absorb significantly large quantity visible radiation than white. back side coins - emissivity. Eventually the object will reach thermal equilibrium, with the absorbed energy from incoming radiation being equal to the energy emitted as outgoing radiation.
I. Organizational moment
The other two factors are geometry and input energy. Per Kirchhoff's radiation law, emissivity and absorptivity at any given frequency are equal. For an ideal gray body, both absorbance and emissivity are constant, regardless of frequency and temperature. All perfect gray bodies with the same geometry and subject to the same incoming radiation will eventually reach the same equilibrium temperature.
Heat transfer in nature is carried out with the help of heat conduction, convection and radiation (radiation absorption and emission).
The mechanism of heat conduction is actually explained in the previous paragraph. Let's take another example. When the end of a metal rod is heated, its molecules begin to move faster, i.e., the internal energy of this end increases. Since the molecules move more slowly at the other end of the rod, inside the rod, with the help of the chaotic movement of atoms and electrons, internal energy is transferred from the hot to the cold end. The transfer of internal energy from one part of a substance to another, due to the chaotic movement of molecules and other particles of a substance, is called thermal conductivity.
So we need something else to explain why black objects get hotter than white ones. The answer is that absorbance and emissivity depend on frequency and temperature for real objects. Ideal gray bodies do not exist. They, if necessary, are suitable for approaching. "Black" and "white" refer to reflectivity in the visible range. An object white color can be very black in the thermal infrared. An object that is distinctly white but thermally black does not heat up as much as an object that would be visibly and thermally black.
Among various kinds metals have the best thermal conductivity. This is due to the fact that they contain free electrons. We also note that the thermal conductivity of a substance in the solid state is greater than in the liquid state, and in the liquid state it is greater than in the gaseous state.
Consider the essence of convection. To show the poor thermal conductivity of water, usually a vessel of water is heated from above. At the same time, the water can boil at the top, but remain cold at the bottom. However, if the vessel is heated from below, then the water is heated evenly throughout the volume. This is explained by the fact that water expands when heated and its density decreases. If the heated water is at the bottom, then the upper, denser layers of water descend under the action of gravity and displace the warm water up. This mixing of water will continue until all the water boils. The heat transfer that occurs when unevenly heated layers of liquid or gas are mixed under the action of gravity is called convection. It is easy to see that convection is absent in a spacecraft in a state of weightlessness.(Consider why the freezer in refrigerators is reinforced at the top instead of the bottom.)
The science behind the design of a sleeping bag is at once simple and yet very complex. Over the years, the design of the sleeping bag has changed and evolved to incorporate the latest technological breakthroughs and use the latest innovative fabrics and materials available. Recent advances in sleeping bag technology include waterproof down insulation, ultralight materials and breathable vapor barriers. Complex as technology can become, the goal in sleeping bag design is very simple.
The design of a sleeping bag boils down to one ultimate goal: to trap dead air around the body so that it doesn't heat up and reduce the body's body heat. Two major factors at play in sleeping bag design reduce heat transfer while creating thermal insulation. Everything else is just marketing.
It may seem that convection cannot be considered heat transfer, since it is associated with the work of gravity. However, during convection, an increase in the internal energy of a liquid or gas occurs only due to the heat supplied from outside, and the effect of gravity is reduced only to accelerating the uniform heating of the liquid or gas. The action of gravity during convection does not give an additional contribution to the internal energy of a liquid or gas. Therefore, convection is referred to as heat transfer.
Heat exchange between the Sun and the Earth is carried out by means of electromagnetic radiation. Electromagnetic radiation is created by the movement of electric charges and increases sharply with increasing temperature. The radiation of a body, which is determined only by its temperature, is called thermal radiation.
The process of radiation occurs due to the internal energy of the body . When radiation is absorbed by some other body, the internal energy of the body increases due to the energy of the absorbed radiation.Thus, by means of radiation, energy is transferred from more heated bodies to less heated ones. This type of heat transfer occurs even in the absence of matter between the bodies.
