The wavelength at which the maximum energy occurs. Laws of black body radiation
Solving problems in physics, quantum optics
Problem 536. Determine which wavelength corresponds to the maximum spectral density of energy luminosity (r λ, T)max equal to 1.3 * 10 11 W / m 3
The solution of the problem.
Tasks for performing independent and control works, quantum optics
1. The Fe energy flux emitted from the viewing window of the melting furnace is 34 W. Determine the temperature T of the furnace if the opening area S = 6 cm2. (Answer: 1kK).
Let's look at some continuous spectra taken with a tungsten anode. The potentials used to accelerate the electron beam are indicated next to the corresponding curve. 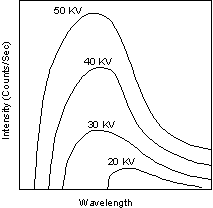
The continuous spectrum is simply a curve of counts per second compared to the X-ray wavelength, i.e. intensity compared to 1. Note that all curves have in common the fact that there is a minimum wavelength below which no X-ray emission is observed. Curiously, this value does not depend on the anode material.
2. The temperature T of the upper layers of the Sirius star is 10 kK. Determine the energy flux Fe radiated from the surface area S = 1 km2 of this star. (Answer: 56.7 GW).
3. The temperature of the upper layers of the Sun is 5.3 kK. Assuming the Sun to be a black body, determine the wavelength m, which corresponds to the maximum spectral density of the energy luminosity of the Sun. (Answer: 547 nm).
To understand this phenomenon, remember the chapter on the photoelectric effect. Therefore, the emitted x-ray beam must have a maximum energy equal to the energy of the incident electron. That is, the continuous spectrum is limited by the wavelength associated with the maximum energy of the electron.
By replacing the tungsten target with a molybdenum target and keeping the rest of the experimental conditions, the result shown below is obtained. 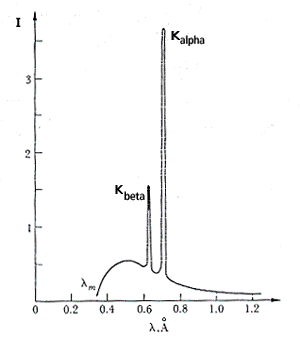
Given that the only difference between one measure and the other is target substitution, it is reasonable to assume that the peaks are due to the molybdenum anode.
4. When the thermodynamic temperature T of a black body doubles, the wavelength m, which accounts for the maximum spectral density of energy luminosity, decreased by = 400 nm. Determine the initial and final temperatures T1 and T2. (Answer: 3.62 kK; 7.24 kK).
5. The temperature T of a black body is 2 kK. Determine: 1) spectral density of energy luminosity (r, Т) for the wavelength = 600 nm; 2) energy luminosity Re in the wavelength range from 1 = 590 nm to 2 = 610 nm. Assume that the average spectral density of the energy luminosity of the body in this interval is equal to the value found for the wavelength = 600 nm. (Answer: 30 MW/m2∙mm; 600 W/m2).
These peaks represent the characteristic X-ray spectrum of molybdenum. In this lesson, the concept of an electromagnetic wave, in particular, part of the electromagnetic spectrum, consisting of thermal radiation, will be considered, introducing the concept of an ideal black body and its characteristics. In addition, the radiation properties of materials, such as emissivity, absorption coefficient, reflection coefficient, and transmittance, will depend on their temperature and wavelength dependence.
Irradiation is another heat transfer phenomenon, particularly related to the internal energy of the body. If, for example, warm body placed in an empty chamber whose walls are at room temperature, we will notice that the body will lose its heat until it reaches thermal equilibrium with the environment. The transfer of heat between the body and the chamber occurs during irradiation, since the transfer takes place in a vacuum. This is a very important feature, precisely because radiation is different from the other two heat transfer phenomena, since it does not require the presence of a medium.
5. For a certain body, its emissivity is nonzero only in the wavelength range . Find the energy luminosity of the body if in the specified range the emissivity of the body is equal to a constant value .
6. The intensity of sunlight near the Earth's surface is about 0.1 W/cm2. The radius of the Earth's orbit is R3=1.5x108 km. Radius of the Sun RC=6.96x108 m. Find the temperature of the surface of the Sun.
In addition, the transmission is faster because it occurs at the speed of light and does not undergo vacuum attenuation, so it can occur as in solids Oh, and in liquids and gases. While heat transfer by conduction or convection occurs in the direction of decreasing temperatures from a medium at a higher temperature to one at a lower temperature, radiative transfer between two bodies can also occur in the presence of a colder separation agent than both bodies.
In addition, if we have a radiation source, the irradiation will be different depending on the relative position before it is cancelled. The theoretical basis of irradiation is based on the concept of an electromagnetic wave or electromagnetic radiation, representing the energy emitted by a substance as a result of changes in the electronic configurations of molecules or atoms of elements.
7. The intensity of solar radiation passing through the atmosphere in summer is approximately 130 W/m2. At what distance should one stand from an electric heater with a power of 1 kW in order to feel the same intensity of radiation. Assume that the electric heater radiates equally in all directions.
8. The sun radiates energy at a speed of 3.9.1026 J/s. What is the intensity of solar radiation near the Earth's surface? The distance from the Earth to the Sun is 150 million km.
The frequency of an electromagnetic wave depends on the source itself and does not depend on the medium through which the wave propagates. The frequency corresponding to the number of oscillations per second may vary depending on the source. Electromagnetic radiation is considered as the propagation of a series of discrete packets called photons, or such as.
Before talking about thermal radiation, you should refer to those that are electromagnetic radiation. The electromagnetic radiation that is identified in the transfer of heat is thermal radiation, that is, the energy radiation of bodies to transfer heat.
9. In low temperature physics, refrigerants are widely used: liquid helium, whose temperature is 4.2 K, and liquid nitrogen, which has a temperature of 77K. What wavelengths account for the maximum power of thermal radiation of cavities filled with these liquids. To which region of the electromagnetic spectrum do these radiations belong?
10. What is the power of thermal radiation of a body heated to a temperature of 500 С, the emissivity of which is 0.9, the area of the radiating surface is 0.5 m2?
Light is the visible part of the electromagnetic spectrum and consists of small bands of color ranging from violet to red. The color of a surface, for example, depends on its ability to reflect certain wavelengths. A surface appears red if it reflects red radiation while absorbing the rest of the visible radiation. The surface that reflects all the light is white and the surface that absorbs all the light is black. The sun is the main source of light, and the electromagnetic radiation emitted is called solar radiation and is almost half light, and for the rest, ultraviolet or infrared radiation.
11. What is the power of thermal radiation of the human body, located at normal temperature 34 С? The body surface area is 1.8 m2.
12. The power of thermal radiation of a body at a certain temperature is 12 mW. What will be the radiation power of the same body if its temperature is doubled?
13. The maximum spectral power of radiation of a completely black body falls on a wavelength of 25 microns. Then the body temperature is increased so that the total radiation power of the body is doubled. Find: a) new body temperature; b) the wavelength at which the maximum spectral density of radiation falls.
Therefore, only thermal radiation, which is also called simple irradiation, is considered in the heat transfer study. Since electrons, atoms and molecules of solids, liquids and gases are constantly moving above absolute zero, irradiation is a three-dimensional phenomenon. For opaque solids, such as metals, wood radiation emitted from interior areas cannot reach the surface, and radiation is usually absorbed at the surface.
It should be noted that such surfaces may differ significantly from their radiation characteristics if paint is applied to them. In summary, the frequencies perceived by the human eye as visible light represent only a small fraction of the known electromagnetic waves with a wavelength λ between 400 and 700 nanometers.
14. A 100 W light bulb has a tungsten filament with a diameter of 0.42 mm and a length of 32 cm. The effective absorptivity of the tungsten filament is 0.22. Find the filament temperature.
15. The outer space of our Universe is filled with background cosmic radiation left over from the Big Bang. The wavelength at which the maximum spectral density of this radiation falls is 1.073 mm. Find: a) the temperature of this radiation; b) the power of this radiation that falls on the Earth.
Below are descriptions of the various electromagnetic waves that make up the spectrum. They are responsible for electromagnetic pollution caused by high voltage power lines. Radio waves Radio waves are used primarily in radio transmissions and in particular for cellular telephony.
Microwaves Microwaves are used primarily in thermal applications such as microwave ovens or for communications and radar systems. Infrared infrared radiation is produced by hot bodies where atoms are excited by impacts caused by thermal mixing. If they are absorbed by a molecule, those with enough energy to cause an oscillatory motion, resulting in an increase in temperature. Infrared radiation is used in medicine for physical therapy and, in research, to study the vibrational energy levels of molecules.
16. Determine the radius of a distant star according to the following data: the intensity of the radiation of this star reaching the Earth is 1.71012 W / m2, the distance to the star is 11 light years, the temperature of the surface of the star is 6600 K.
17. A surface of 10 cm2 heated to 2500 K emits 6700 J in 10 s. What is the absorption coefficient of this surface?
Visible light The field of visible light is very close to the entire spectrum of radiation, although it is very important for living organisms, since the eye of most of them is sensitive to this radiation. Ultraviolet The main sources of ultraviolet waves are the sun, lightning and the arc of electric welders. Much of the ultraviolet radiation produced by the sun is absorbed by the atmosphere, causing the ozone formation reaction that is essential for life on Earth, as this radiation is large quantities is lethal.
Everyone knows the problem of depletion of the ozone layer mainly by chlorofluorocarbons. Plus ultraviolet rays are high frequency, the more they are harmful to living beings; not so much because it increases their penetrating power in tissues, especially since it approaches the wavelengths that resonate molecular bonds, leading them to break. The main application of ultraviolet radiation is sterilization.
18. The spiral of a 25 W light bulb has an area of 0.403 cm2. Incandescent temperature 2177 K. What is the absorption coefficient of tungsten at this temperature?
19. A tungsten filament is heated in a vacuum with a current of 1 A to a temperature of 1000 K. What current must be passed through the filament so that its temperature becomes 3000 K? Ignore energy losses due to thermal conductivity and changes in the linear dimensions of the thread.
X-ray radiation Their main application is in the field of medicine. Their penetrating power is very high, so that they can cross the human body and reach the internal organs. The absorption of x-rays is different in the tissues of the human body and especially in the bones; therefore, the X-ray radiation flowing through the body differs depending on the intersection of the tissue and the photographic plate, more or less impressive.
γ Rays These radiations are typical of cosmic rays, but do not reach the Earth's surface because they are first filtered out of the atmosphere. They are also extremely harmful to human cells as they lead to the destruction of molecular structures. Prolonged exposure to γ-rays caused by a nuclear reaction can be fatal even if energy transport is low.
20. The thermostat consumes 0.5 kW power from the network. The temperature of its inner surface, determined by radiation from an open round hole with a diameter of 5 cm, is 700 K. How much power is dissipated by the outer surface of the thermostat?
21. A tungsten filament with a diameter of d1=0.1 mm is connected in series with another similar filament. The filaments are heated in a vacuum by an electric current, so that the first filament has a temperature T1=2000 K and the second one T2=3000 K. What is the diameter of the second filament?
The emission of radiation and the way the organism interacts are properties that depend on the surface treatments of the bodies. To simplify the task, simplification was introduced through the concept of a blackbody. An ideal blackbody, called a blackbody, is defined as a body whose function is to be used as a reference to incident radiation, regardless of direction and wavelength. Since the blackbody radiates uniformly radiant energy in all directions, it is a diffuse transmitter, i.e. works regardless of direction.
Let us now study the energy emitted by the body: only being at a given temperature, it will become a source of electromagnetic radiation. At the same temperature, different bodies radiate different energies. However, it is not possible for an outlier to exceed a certain value; the blackbody is the source that can reach this limiting emission. In the general case, the spectrum is a comparison of the radiation properties of real bodies. A black body is an ideal emitter emitter and absorber, because it emits maximum radiation for each temperature and wavelength, and absorbs all the radiation of a material at a certain temperature depending on the wavelength, has a curvilinear structure with different maximum and minimum; the spectrum of the black spectrum spectrum is obtained from the envelope of the infinite spectra of different bodies, since, as mentioned earlier, no body at any wavelength can radiate more energy than it does.
22. Taking the positive arc crater as a black body, determine the ratio of the radiation power in the wavelength range from 695 nm to 705 nm to the total radiation power. The arc crater temperature is 4000 K.
23. The radiation power measured in the interval 1=0.5 nm near the wavelength corresponding to the radiation maximum MAX is equal to the radiation power in the interval 2 near the wavelength =2MAX. Determine the width of the interval 2.
The black body is an abstraction because it cannot exist strictly by nature, although it is possible to reconstruct an object in the laboratory whose emissivity is close to that of a black body. The strength of the radiation emitted by a black body per unit surface is determined by the ratio.
Note in this report that the emissive power of a black body is proportional to the fourth power of absolute temperature. Even though a black body will appear black, a distinction must be made between a perfect black body and a black surface. A surface that absorbs light appears black to the eye as a surface that reflects it appears completely white. Since visible radiation occupies a very low part of the spectrum, it is impossible to judge whether an area approaches a blackbody in a single visual observation.
24. The temperature T of a completely black body is 2kK. Determine: 1) the spectral density of the radiation flux r) for the wavelength =600 nm; 2) radiation power density Re in the wavelength range from 1=590 nm to 2=610 nm. Assume that the average spectral density of the radiation flux in this interval is equal to the value found for the wavelength =600 nm.
25. The temperature T of the upper layers of the Sirius star is 10,000 K. Determine the energy flux Ф radiated from a surface area S = 1 km2 of this star.
26. The temperature T of the upper layers of the Sun is 5300 K. Assuming the Sun to be an absolutely black body, determine: a) the wavelength m, which corresponds to the maximum spectral radiation density rMAX); b) the value of rMAX).
27. A tungsten filament is heated in a vacuum with a current of 1 A to a temperature of 1000 K. What current must be passed through the filament so that its temperature becomes 3000 K? The absorption coefficients of tungsten and its resistivity, corresponding to temperatures Т1 and Т2, are
28. A body with a mass m=10 g and a surface S=200 cm2, having a temperature T0=600K, is placed in a vacuum. Determine to what temperature T the body will cool in time t=30 s, if the absorptivity of the body surface =0.4, and specific heat c = 350J/kg.K.
29. Find the solar constant I, that is, the amount of radiant energy sent by the Sun per unit time through a unit area located perpendicular to the sun's rays and located at the same distance from the Sun as the Earth. The surface temperature of the Sun is T=5800 K., the distance from the Earth to the Sun is L=1.51011 m.
30. Determine how long it takes for a copper ball placed in a vacuum to cool from T1=500 K to T2=300 K. The ball radius R=1 cm, surface absorptivity =0.8, specific heat capacity of copper c=0.39 J/g.K , specific gravity of copper =8.93 g/cm3.
31. Is it possible to measure, on a sensitive scale, which allows one to note a change in mass by 10-40%, an increase in the mass of a piece of tungsten (a very refractory metal) when it is heated from 0 to 33000C (average specific heat capacity can be considered equal to C = 120 J / kg deg) ? (Answer: The relative increase in a unit mass during heating will be 4.4.10-12, which is hundreds of times less than the value available for measurement).
32. Explain why in an unheated room the temperature of all bodies is the same.
33. Energy luminosity of a black body Re = 10 kW/m2. Determine the wavelength corresponding to the maximum spectral density of the energy luminosity of this body. (Answer: 4.47 microns).
34. Determine how and how many times the radiation power of a black body will change if the wavelength corresponding to the maximum of its spectral density of energy luminosity has shifted from λ1 = 720 nm to λ2 = 400 nm. (Answer: It will increase by 10.5 times).
35. As a result of heating the black body, the wavelength corresponding to the maximum spectral density of energy luminosity shifted from λ1 = 2.7 microns to λ2 = 0.9 microns. Determine how many times increased: 1) the energy luminosity of the body; 2) the maximum spectral density of the energy luminosity of the body. The maximum spectral density of the energy luminosity of a black body increases according to the law rλT = CT5, where C = 1.3.10-5 W/(m3.K5). (Answer: 1) 81 times; 2) 243 times).
36. Determine which wavelength corresponds to the maximum spectral density of energy luminosity (rλT)max, equal to 1.3.1011 (W / m2) / m (see problem 5.12). (Answer: 1.83 µm).
37. Assuming that heat losses are due only to radiation, determine how much power must be supplied to a copper ball with a diameter of d \u003d 2 cm, so that at a temperature environment t0 = -13 °C to maintain its temperature equal to t = 17 °C. Take the absorption capacity of copper AT = 0.6. (Answer: 0.107 W).
38. Calculate the true temperature T of a hot tungsten tape if the radiation pyrometer shows a temperature of Trad = 2.5 kK. Assume that the absorption capacity for tungsten does not depend on the radiation frequency and is equal to a=0.35.
39. Calculate the energy emitted during the time t=1 min from the area S=l cm2 of a completely black body, the temperature of which is T=1000 K.
40. A black body has a temperature T1 = 500 K. What will be the temperature T2 of the body if, as a result of heating, the radiation flux increases n = 5 times?
41. The wavelength, which accounts for the maximum radiation energy of a completely black body, m=0.6 microns. Determine the temperature T of the body.
42. The temperature of a completely black body T \u003d 2 kK. Determine the wavelength m, which accounts for the maximum radiation energy, and the spectral density of energy luminosity (r,T)max for this wavelength.
43. Determine the maximum spectral density (r, T)max of energy luminosity, calculated per 1 nm in the radiation spectrum of a black body. Body temperature T=1 K.
44. Determine the temperature T and the energy luminosity Re of a completely black body if the maximum radiation energy falls on the wavelength m = 600 nm.
45. A stream Fe = 4 kJ / min is emitted from the viewing window of the furnace. Determine the temperature T of the oven if the area of the window is S=8 cm2.
46. The radiation flux of a completely black body Fe \u003d 10 kW. The maximum radiation energy falls on the wavelength m=0.8 µm. Determine the area S of the radiating surface.
47. How and how many times will the radiation flux of a completely black body change if the maximum radiation energy moves from the red border of the visible spectrum (m1=780 nm) to the violet (m2=390 nm)?
48. Determine the absorption capacity a of a gray body, for which the temperature measured by a radiation pyrometer is Trad = 1.4 kK, while the true temperature T of the body is 3.2 kK.
49. A muffle furnace that consumes power ^ P \u003d 1 kW has an opening with an area of \u200b\u200bS \u003d 100 cm2. Determine the fraction of the power dissipated by the furnace walls if the temperature of its inner surface is 1 kK.
50. The average energy luminosity ^ R of the Earth's surface is 0.54 J / (cm2 min). What should be the temperature T of the Earth's surface, if we conditionally assume that it radiates as a gray body with a blackness coefficient a = 0.25?
51. An absolutely black body has a temperature of 500 K. What will be the temperature of the body if, as a result of heating, the radiation flux increases 5 times? Based on Planck's formula, graphically depict the initial and final radiation spectra.
52. The temperature of a completely black body is 2000 K. Determine the wavelength at which the maximum radiation energy spectrum falls, and the spectral density of energy luminosity for this wavelength.
53. Determine the temperature and energy luminosity of a completely black body if the maximum energy of the radiation spectrum falls at a wavelength of 600 nm.
54. A stream of 4 kJ / min is emitted from the viewing window of the furnace. Determine the temperature of the furnace if the area of the window is 8 cm2.
55. The radiation flux of a completely black body is 10 kW, and the maximum of the radiation spectrum falls on a wavelength of 0.8 microns. Determine the area of the emitting surface.
56. How and how many times will the radiation flux of a completely black body change if the maximum of the visible radiation spectrum moves from the red edge of the spectrum at 780 nm to the violet at 390 nm?
57. Determine the intensity of solar radiation (radiation flux density) near the Earth outside its atmosphere, if in the spectrum of the Sun the maximum spectral density of energy luminosity falls at a wavelength of 0.5 microns.
58. Calculate the energy (kWh) radiated per day from an area of 0.5 m2 of a heater whose temperature is 700C. Consider that the heater radiates as a gray body with an absorption coefficient of 0.3.
59. The average energy luminosity of the Earth's surface is 0.54 J / (cm2min). What is the average temperature of the Earth's surface, assuming that it radiates as a gray body with an absorption coefficient of 0.25?
60. A furnace consuming a power of 1 kW has an opening with an area of 100 cm2. Determine the fraction of power dissipated by the furnace walls if the temperature of its inner surface is 1000 K.
61. When a completely black body cools down, the maximum of its emission spectrum shifted by 500 nm. By how many degrees has the body cooled down? The initial body temperature is 2000 K.
62. An absolutely black body in the form of a ball with a diameter of 10 cm emits 15 kcal / min. Find the temperature of the ball.
63. An absolutely black body has the form of a cavity with a small hole, the diameter of which is 1 cm. The heating of the body is carried out by an electric spiral that consumes a power of 0.1 kW. Determine the value of the equilibrium temperature of the radiation emanating from the hole if the walls of the cavity dissipate 10% of the power.
64. What mass does the Sun lose for radiation in 1 s? Estimate also the time during which the mass of the Sun will decrease by 1%.
65. Determine to what temperature a ball with a diameter of 10 cm with an absolutely black surface will cool due to radiation after 5 hours, if its initial temperature is 300 K. The density of the ball material is 104 kg / m3, the heat capacity is 0.1 cal / (g deg ). Neglect environmental radiation.
66. Estimate the thermal power emitted by a space station whose surface area is 120 m2, temperature - (- 500C), and absorption coefficient - 0.3. Neglect environmental radiation.
67. What is the power radiated from the window if the temperature in the room is 200C and the outside temperature is 00C? The absorption coefficient of the window is considered equal to 0.2, and its area is 2 m2.
68. Determine the power required to incandescent a tungsten filament of an electric lamp with a length of 10 cm and a filament diameter of 1 mm to a temperature of 3000 K. Ignore heat losses due to thermal conductivity and convection.
69. A tungsten filament is heated in a vacuum with a current of 1.0 A to a temperature of 1000 K. At what current strength will the filament heat up to a temperature of 3000 K? The corresponding absorption coefficients are 0.115 and 0.334, and the temperature coefficient of resistivity is assumed to be 4.103 Ohm m/deg.
70. To what temperature does a small spherical metal meteorite heat up from sunlight in near-Earth outer space?
71. Two balls of different diameters and made of the same material are heated to the same temperature, so that part of their emission spectrum is in the visible range. The balls are at the same distance from the observer. Which ball (larger or smaller) will be seen better and why?
72. If you look inside the cavity, the temperature of the walls of which is maintained constant, then no details can be seen inside. Why?
73. Betelgeuse - a star in the constellation Orion - has a surface temperature well below the sun. However, this star radiates much more energy into space than the Sun. Explain how it could be.
74. A 100 W light bulb emits only a few percent of its energy in the visible range. Where does the rest of the energy go? How can the radiation energy in the visible range be increased?
75. Any body whose absolute temperature is not equal to zero radiates energy, however, not all bodies are visible in the dark. Why?
76. Do all hot bodies obey the law: where the coefficient k depends on the material of the body and on its temperature?
77. The power of thermal radiation of the human body is approximately 1 kW. Why then is a person not visible in the dark?
78. Two identical bodies have the same temperature, but one of them is surrounded by colder bodies than the other. Will the radiation powers of these bodies be equal under these conditions?
79. Why does the color of the body change when heated?
80. How will the wavelength corresponding to the maximum emissivity of an absolutely black body change if this body is surrounded by an absolutely absorbing shell with a larger surface than that of the body, but radiating the same power as the body?
81. The temperature of a completely black body has doubled. How many times has its energy luminosity increased?
82. Why do the unlit windows of houses seem dark to us during the day, although it is light in the rooms of the houses?
83. How many times will the energy luminosity of a completely black body change if its temperature is doubled?
84. How many times will the radiation power of a completely black body change if its surface area is doubled?
85. The wavelength, which accounts for the maximum emissivity of a completely black body, has halved. How will the area bounded by the curve describing the dependence of the emissivity on the radiation wavelength change in this case? This area will: a) decrease? b) increase? How many times?
86. How will the total amount of radiation energy of an absolutely black body change if one half of it is cooled twice, and the temperature of the second half is reduced by half?
87. A black body is heated to a temperature of T = 1000 K. At what wavelength is the radiation power maximum?
88. A black body is heated to a temperature of T = 1000 K. At what frequency is the radiation power maximum?
89. A ball with a radius R = 1 cm is heated to a temperature T = 1000 K. Considering the radiation of the ball as black, determine the total power radiated by this ball into space.
90. A thin disk with a radius of R = 1 cm is heated to a temperature of T = 1000 K. Assuming the radiation of the disk to be black, determine the total power radiated by this disk into space.
91. A ball with a radius of R = 1 cm is heated to a temperature of T = 1000 K. Assuming the radiation of the ball to be black, determine what power the same ball will absorb, located at a distance l = 10 m from the heated one.
92. A thin disk with a radius of R = 1 cm is heated to a temperature of T = 1000 K. Considering the radiation of the disk to be black, determine how much power the same disk will absorb, located at a distance l = 10 m from the heated one so that their axes coincide and the planes are parallel.
93. Considering the Sun and the Earth as absolutely black bodies, determine to what temperature the Earth will heat up under the influence of sunlight. The temperature of the Sun's surface is assumed to be Т=6000 K, the distance from the Sun to the Earth is L=1.51011 m. The radius of the Sun is RC= 7108m. Radius of the Earth RЗ=6.4106 m. Neglect the influence of the earth's atmosphere.
94. In the upper layers of the atmosphere, the intensity of solar radiation is 1.37103 W/m2. Neglecting the influence of the atmosphere and assuming that the Earth radiates as a completely black body, determine the temperature to which the Earth will heat up under the action of solar radiation.
95. In 1983, an infrared telescope mounted on a satellite discovered a cloud of solid particles around the star Vega, the maximum radiation power of which was at a wavelength of 32 microns. Considering the radiation of the cloud as black, determine its temperature.
96. Calculate the wavelength that accounts for the maximum radiation power and determine the region of the electromagnetic spectrum for: a) background cosmic radiation having a temperature of 2.7 K; b) a human body with a temperature of 34 С; c) an electric light bulb, the tungsten filament of which is heated to 1800K; d) the Sun, whose surface temperature is 5800 K; e) a thermonuclear explosion occurring at a temperature of 107K; f) the Universe immediately after the Big Bang at a temperature of 1038 K.
97. What frequency should the receiving circuit of a radio telescope be tuned to in order to detect background cosmic radiation, the temperature of which is 2.7K?
98. In the cavity, the walls of which are heated to a temperature of 1900K, a small hole with a diameter of 1 mm is drilled. What will be the flux of radiation energy through this hole?
99. The temperature of a tungsten filament in a light bulb is usually about 3200 K. Assuming that the filament radiates as an absolutely black body, determine the frequency at which the maximum spectral power of the radiation falls.
100. The temperature of a tungsten filament in a light bulb is usually about 3200 K. Assuming that the filament radiates as a completely black body, determine the radiation power of the light bulb. The diameter of the tungsten filament is 0.08 mm, its length is 5 cm.
101. The oven, inside which the temperature is 215 С, is located in a room in which a constant temperature of 26.2 С is maintained. A small hole with an area of 5.2 cm2 was made in the furnace. What is the radiation power from this hole?
102. A 100 W light bulb spiral is a tungsten filament with a diameter of 0.28 mm and a length of 1.8 m. Considering the radiation of the spiral to be black, calculate: a) the working temperature of the filament; b) the time after which the thread will cool down to 500 С after the light bulb is turned off. The specific gravity of tungsten is 19.3 g/cm3, its heat capacity is 0.134 J/g С.
103. The spectral density of radiation of a completely black body at a wavelength of 400 nm is 3.5 times greater than at a wavelength of 200 nm. Determine body temperature.
104. The spectral density of radiation of a completely black body at a wavelength of 400 nm is 3.5 times less than at a wavelength of 200 nm. Determine body temperature.
105. Radiation power of a completely black body P = 100 kW. What is the area of the radiating surface of the body if the wavelength at which the radiation maximum falls is 700 nm?
106. Due to a change in body temperature, the maximum of its spectral energy luminosity has moved from a wavelength of =2.5 microns to =0.125 microns. Assuming the body is absolutely black, determine how many times has changed: a) body temperature; b) the maximum value of the spectral energy luminosity; c) integrated energy luminosity.
107. Maximum spectral energy luminosity of an absolutely black body (]max=4.16х1011 W/m2). What wavelength is it?
108. Calculate the spectral energy luminosity of a black body heated to 3000 K for a wavelength of 500 nm.
109. Determine the values of the spectral powers of radiation of a black body for the following wavelengths: =MAX, =0.75MAX, =0.5MAX, =0.25MAX. Body temperature 3000 K.
110. The radiation power P of a ball with a radius R = 10 cm at a certain constant temperature T is equal to 1 kW. Find this temperature, considering the ball as a gray body with an absorption coefficient =0.25.
111. There are two absolutely black sources of thermal radiation. The temperature of one of them is T1=2500 K. Find the temperature of the other source if the wavelength corresponding to the maximum of its emissivity is =0.50 µm greater than the wavelength corresponding to the maximum of the emissivity of the first source.
112. How much energy does the Sun radiate in 1 minute? The radiation of the Sun is considered close to the radiation of a completely black body. The temperature of the surface of the Sun is taken equal to 58000 K. The radius of the Sun is Rc=7.108 m.
113. An absolutely black body is at a temperature T1=29000K. As a result of the cooling of this body, the wavelength, which accounts for the maximum spectral density of energy luminosity, has changed by =9 μm. To what temperature T2 did the body cool?
114. A satellite in the form of a ball moves around the Earth at such a height that the absorption of sunlight can be neglected. The diameter of the satellite is d=40 m. Assuming that the surface of the satellite completely reflects light, determine the pressure force F of the sunlight on the satellite. Radius of the Sun Rc=7108m. The distance from the Earth to the Sun is L=1.5.1011m. The temperature of the surface of the Sun T=60000K.
115. With an increase in the temperature of an absolutely black body, its integral energy luminosity increased 5 times. How many times did the wavelength change, which accounts for the maximum spectral density of radiation?
116. The radiation power of a completely black body is 34 kW. Find the temperature of this body if it is known that its surface is 0.6 m2.
117. Find how much energy an absolutely black body emits from 10 cm2 of surface in 1 minute if it is known that the maximum spectral density of its energy luminosity falls on a wavelength of 4840 A.
118. Find the temperature of the furnace, if it is known that from a hole in it with a size of 6.1 cm2 radiates in 1 min 50 J. Consider the radiation close to the radiation of a completely black body.
119. Determine the temperature T at which the energy luminosity R of a completely black body is 10 kW / m2.
120. The radiation of the Sun in its spectral composition is close to the radiation of an absolutely black body, for which the maximum emissivity falls on a wavelength of 0.48 microns. Find the temperature of the surface of the Sun.
121. Determine the relative increase R / R of the radiation power of a completely black body with an increase in its temperature by 1%.
122. Determine the energy W radiated over time t=1 min from a viewing window with an area S=8 cm2 of the melting furnace if its temperature is T=1200K.
123. Determine the temperature T of a completely black body, at which the maximum spectral density of radiation is rMAX); falls on the red border of the visible spectrum (1=750 nm).
124. The average value of energy lost as a result of radiation from 1 cm2 of the Earth's surface during 1 minute is 5.4x10-8 J. What temperature should an absolutely black body emitting the same amount of energy have?
125. The temperature of a hair of a 15 W light bulb powered by alternating current fluctuates so that the difference between the highest and lowest incandescent temperatures of the tungsten filament is 80 ° C. How many times does the total radiation power change due to temperature fluctuations if its average value is 2300K? Accept that tungsten radiates as a black body.
126. The muffle furnace consumes power P = 0.5 kW. The temperature of its inner surface with an open hole with a diameter of d = 5 cm is 700 C. What part of the power consumption is dissipated by the walls?
127. During the operation of radio tubes, the anode is heated due to its bombardment with electrons. Assuming that the anode dissipates energy only in the form of radiation, determine the permissible anode current in a lamp operating at a voltage of 40 V. The nickel anode has the shape of a cylinder 4 cm long and 1 cm in diameter. The permissible temperature to which the anode can be heated is 1000K. At this temperature, nickel emits only 20% of the radiation power of a black body.
128. A grate with an area of 2 m2 is surrounded by iron walls. The temperature of the coal on the grate is 1300K, the temperature of the walls is 600K. The absorption coefficients of coal and oxidized iron can be considered equal to 0.9. Calculate the amount of heat transferred by radiation from the grate to the walls in 1 hour.
129. Inside solar system at the same distance from the Sun as the Earth, there is a particle of a spherical shape. Assuming that the Sun radiates as an absolutely black body with a temperature of 6000K and that the particle temperature is the same at all its points, determine its temperature if the particle has the properties of a gray body. The distance from the Sun to the Earth is L=1.51011 m. The radius of the Sun is RC= 7108 m.
130. Inside the solar system, at the same distance from the Sun as the Earth, there is a spherical particle. Assuming that the Sun radiates as an absolutely black body with a temperature of 6000 K and that the temperature of the particle at all its points is the same, determine its temperature if the particle absorbs and emits only rays with a wavelength of 500 nm. The distance from the Sun to the Earth is L=1.51011 m.
131. Inside the solar system, at the same distance from the Sun as the Earth, there is a spherical particle. Assuming that the Sun radiates as an absolutely black body with a temperature of 6000 K and that the temperature of the particle at all its points is the same, determine its temperature if the particle absorbs and emits only rays with a wavelength of 5 μm. The distance from the Sun to the Earth is L=1.51011 m.
132. Passing aphelion, the Earth is 3.3% further from the Sun than when it passes perihelion. Taking the earth as a gray body with an average temperature of 288 K, determine the temperature difference that the earth has at aphelion and perihelion.
133. In a light bulb, a tungsten filament with a diameter of d = 0.05 cm heats up during operation to a temperature of T1 = 2700 K. How long after the current is turned off will the temperature of the filament drop to T2 = 600 K? When calculating, assume that the filament radiates as a gray body with an absorption coefficient of 0.3. The specific gravity of tungsten is 19.3 g/cm3, and the heat capacity is 0.134 J/g C.
134. An electric light bulb that consumes power of 25 W is enclosed in a paper lampshade, having the shape of a ball with a radius of R \u003d 15 cm. To what temperature will the lampshade heat up? Consider that all the power consumed by the lamp goes to radiation and the lampshade radiates as a gray body.
135. An electric light bulb that consumes 100 watts of power is enclosed in a paper lampshade, shaped like a ball with a radius. What is the minimum radius the lampshade should be so that the paper does not catch fire? Consider that all the power consumed by the lamp goes to radiation and the lampshade radiates as a gray body. The ignition temperature of paper is 250°C.
136. Determine the radiation power of 1 cm2 of the surface of an absolutely black body for wavelengths different from the wavelength corresponding to the maximum radiation by 1%. Body temperature is 2000K.
137. Determine the ratio of the radiation powers of 1 cm2 of the surface of a completely black body in the wavelength range from 695 microns to 705 microns (red area) and from 395 microns to 405 microns (section purple). Body temperature is 4000K.
138. The rays of the Sun are collected by means of a lens with a diameter of d = 3 cm on a small hole in the cavity, the walls of which are blackened inside and shiny outside. The opening of the cavity is at the focus of the lens. Determine the temperature inside the cavity. Assume that the intensity of solar radiation passing through the atmosphere is approximately 130 W/m2
139. There are two black emitters with temperatures T1=1000K and T2=500K. What are equal to: a) the ratio of wavelengths max,1 / max,2, which account for the maximum in the emission spectrum; b) the ratio of the maximum emissivity of two bodies rmax1,T1)/rmax2,T2). Show on one graph the qualitative dependence r,T for two emitters.
140. With an increase in the thermodynamic temperature T of an absolutely black body by a factor of 2, the wavelength m, which accounts for the maximum spectral density of radiance, changed by =400 nm. Determine the initial and final temperatures T1 and T2.
141. The distance between the Sun and the planets Venus and Earth, respectively, are RВ=1.1х108 km, RЗ=1.5х108 km. Considering the Earth and Venus as absolutely black bodies, devoid of an atmosphere, determine to what temperature Venus will heat up under the action of sunlight if the Earth heats up to 20°C.
142. The radiation of the Sun in its spectral composition is close to the radiation of an absolutely black body, for which the maximum emissivity falls on the wavelength =0.48 microns. Find the mass lost by the Sun every second due to radiation. Estimate the time it takes for the mass of the sun to decrease by 1%.
143. Determine the wavelength that accounts for the maximum value of the emissivity of a completely black body equal to 6.1011 W / m3.
144. A plate with a black surface is placed perpendicular to the incident rays in a vacuum. Determine the energy E absorbed by 1 cm2 of the plate surface in 1 min if the temperature of the plate surface is set to 500K.
145. The wavelength corresponding to the maximum spectral density of radiation for the Polar Star and the star Sirius are equal, respectively: П=0.35 µm, С=0.29 µm. Calculate the temperature of the surfaces of these stars and the ratio of their integral and spectral (at maximum) powers of radiation from a unit surface of these stars, considering them to be absolutely black bodies.
146. The diameter of the tungsten spiral in a light bulb is d=0.3 mm, the length of the spiral is l=5 cm. At a voltage of 127 V, a current of 0.31 A flows through the bulb. What is the temperature of the spiral if energy is lost only due to thermal radiation. Tungsten absorption coefficient Т=Т, where .
147. Calculate the steady-state temperature of an absolutely black plate located in a vacuum and located perpendicular to the flow of radiant energy 1.4103 W/m2. Determine what wavelength accounts for the maximum spectral density of radiation at the found temperature.
148. Assuming the Sun to be an absolutely black body, find the decrease in the mass of the Sun in 1 year due to radiation. Take the temperature of the surface of the Sun equal to 5800 K.
149. Find the maximum value of the emissivity of a completely black body, if it corresponds to a wavelength =1.45 microns.
150. The temperature of an absolutely black body has increased from T1=500 K to T2=1500 K. How many times has this changed: a) the energy emitted by a unit of body surface per unit of time; b) energy luminosity; c) the maximum value of the emissivity; d) the wavelength at which the maximum spectral density of radiation falls; e) the frequency at which the maximum spectral density of radiation falls?
151. Calculate the true temperature T of a hot tungsten spiral if the radiation pyrometer shows a temperature of TR=2500 K. The absorption coefficient of tungsten does not depend on frequency and is equal to =0.35.
152. Calculate the true temperature T of a hot tungsten coil if the radiation pyrometer shows a temperature of TR=2500 K. The absorption coefficient of tungsten T=T, where ..
153. Inside the solar system, at the same distance from the Sun as the Earth, there is a small flat disk with a radius of R = 0.1 m. Considering the disk as an absolutely black body and assuming that the Sun radiates as an absolutely black body with a temperature of 6000 K, determine the temperature of the disk. The distance from the Sun to the Earth is L=1.5.1011 m.
154. The temperature of a completely black body is 2000 K. Estimate what proportion of the radiated energy flux falls on the visible part of the spectrum (from 400 nm to 700 nm).
155. To what extent would the temperature of the Earth drop in 100 years if solar energy ceased to flow to the Earth? The radius of the Earth is 6400 km; specific heat capacity 200 J/kgK, density 5500 kg/m3; average surface temperature 280 K, absorption coefficient 0.8.
156. The energy luminosity of an absolutely black body is 3 W/cm2. Determine the temperature of the body and the wavelength at which the maximum emissivity of the body falls.
157. After what time would the mass of the Sun be reduced by half due to thermal radiation, if its power remained constant? The temperature of the surface of the Sun is taken equal to 5800K and the Sun is considered to be an absolutely black body.
158. How many times will the energy luminosity of an absolutely black body change in a small range of wavelengths near =5 μm with an increase in body temperature from 1000K to 2000K?
159. An absolutely black body has a temperature of 2000 K. To what temperature did the body cool and how much did the maximum value of the emissivity of the body change if the wavelength, which accounts for the maximum emissivity, changed by 9 microns?
160. A ball with a diameter of d = 1.5 cm, heated to a temperature of T0 = 300 K, was placed in a vessel from which the air was evacuated. The temperature of the vessel is maintained at 77 K. Assuming the surface of the ball to be absolutely black, find after what time its temperature will decrease by half. Ball material density 700 kg/m3, heat capacity C=300 J/kgK.
161. Find the temperature of the tungsten filament of a 25 W incandescent lamp, if the radiating surface area of the filament is S=0.4 cm2, and the absorption coefficient of tungsten is T=T, where K.
162. The hair of an incandescent lamp, designed for voltage U=2 V, has a length l=10 cm and a diameter d=0.03 mm. Assuming that the hair radiates as an absolutely black body, determine the temperature of the thread and the wavelength at which the maximum in the radiation spectrum falls. Specific resistance of hair material =5.510 Ohm. Ignore losses due to thermal conduction.
163. Determine the energy luminosity of a completely black body in the wavelength range corresponding to the visible part of the spectrum (from 0.4 microns to 0.8 microns). The body temperature is 1000 K. Assume that the spectral density of radiation in this range does not depend on the wavelength and is equal to its value at =0.6 µm.
164. Determine the absorptive capacity of a gray body T, for which the temperature measured by a radiation pyrometer is T=1400 K, while the true temperature is T=3200 K.
165. What power must be supplied to a lead ball with a radius of 4 cm to maintain its temperature at t1=27 C, if the ambient temperature is t2=23 C. The absorption capacity of lead is 0.6. Assume that energy is lost only due to radiation.
166. A light filter is placed between the light bulb and the photocell, which transmits radiation in the wavelength range from 0.99 microns to 1.01 microns. At a temperature of a light bulb coil equal to 1500 K, the current through the photocell is 20 mA. Assuming that the current through the photocell is proportional to the power of the radiation incident on it, determine how many times this current will change if the temperature of the light bulb spiral is increased to 2000 K.
167. Estimate what fraction of the power of a 100 watt light bulb falls on the visible part of the spectrum (from 400 nm to 700 nm). Take the temperature of the light bulb filament equal to 2500 K and assume that the light bulb radiates as a completely black body.
168. Electromagnetic radiation inside your eye consists of two components: a) black radiation at a temperature of 310 K and b) visible light, in the form of photons, entering the eye through the pupil. Estimate: a) the total energy of black radiation in the eye; b) the energy of visible radiation in the eye, coming from a 100 W light bulb, if you are at a distance of 2 meters from it. The pupil area is S=0.1 cm2, the diameter of the eyeball is d=3 cm. The light bulb emits only 2% of its power in the visible range (from 400 nm to 700 nm).
169. Calculate the allowable duration of the radiotelephone in the transmitter mode, if the maximum allowable energy load on the biological tissues of the human head at a frequency of 900 MHz is 2 W. hour/m2. Radiation power of the radiotelephone Р=0.5 W. The minimum distance from the radiotelephone antenna to the head is r=5 cm. Assume that the antenna radiates uniformly in all directions.
170. Explain why open windows houses from the side of the streets appear black.
171. A porcelain tea cup on a light background has a dark pattern. Explain why if this cup is quickly removed from the oven, where it was heated to a high temperature, and viewed in the dark, then a light pattern is observed on a dark background.
172. There are two identical aluminum teapots in which the same amount of water is heated to the same temperature. One kettle is sooty and the other is clean. Explain which kettle will cool faster and why.
173. Determine how many times it is necessary to reduce the thermodynamic temperature of a black body so that its energy luminosity Re is weakened by 16 times. (Answer: 2 times).
174. The temperature of the inner surface of a muffle furnace with an open hole of 30 cm2 is 1.3 kK. Assuming that the furnace opening radiates as a black body, determine what part of the power is dissipated by the walls if the power consumed by the furnace is 1.5 kW. (Answer: 0.676).
175. A black body is at a temperature T1 = 3 kK. As the body cools, the wavelength corresponding to the maximum spectral density of energy luminosity changed by Δλ = 8 μm. Determine the temperature T2 to which the body has cooled. (Answer: 323 K).
176. A black body was heated from temperature T1 = 600 K to T2 = 2400 K. Determine: 1) how many times its energy luminosity increased; 2) how the wavelength corresponding to the maximum spectral density of energy luminosity has changed. (Answer: 1) 256 times; 2) decreased by 3.62 µm).
177. The area bounded by the graph of the spectral density of the energy luminosity rλT of a black body, in the transition from thermodynamic temperature T1 to temperature T2, increased 5 times. Determine how the wavelength λmax will change in this case, corresponding to the maximum spectral density of the energy luminosity of a black body. (Answer: It will decrease by 1.49 times).
178. Considering nickel as a black body, determine the power required to maintain the temperature of molten nickel at 1453 ° C unchanged if its surface area is 0.5 cm2. Ignore energy losses. (Answer: 25.2 W).
179. A metal surface with an area of \u200b\u200bS \u003d 15 cm2, heated to a temperature of T \u003d 3000 K, radiates 100 kJ in one minute. Determine: 1) the energy emitted by this surface, considering it black; 2) the ratio of the energy luminosities of this surface and the black body at a given temperature. (Answer: 413 kJ; 0.242).
180. Taking the Sun as a black body, and taking into account that its maximum spectral density of energy luminosity corresponds to a wavelength λ = 500 nm, determine: 1) the temperature of the Sun's surface; 2) the energy emitted by the Sun in the form of electromagnetic waves in 10 minutes; 3) the mass lost by the Sun during this time due to radiation. (Answer: 5800 K; 2.34.1029 J; 2.6.1012 kg).
181. Determine the strength of the current flowing through a tungsten wire with a diameter of d \u003d 0.8 mm, the temperature of which in vacuum is maintained constant and equal to t \u003d 2800 ° C. The surface of the wire is taken as gray with an absorption capacity of AT = 0.343. The specific resistance of the wire at a given temperature ρ = 0.92.10-4 Ohm.cm. The temperature of the medium surrounding the wire t0 = 17 °C. (Answer: 48.8 A).
182. Convert Planck's formula for the spectral density of the energy luminosity of a black body from the variable ν to the variable λ.
183. Using the Planck formula, determine the spectral density of the radiation flux per unit surface of a black body per narrow wavelength interval Δλ = 5nm near the maximum spectral density of energy luminosity if the temperature of the black body is T = 2500K. (Answer: rλTΔλ = 6.26 kW/m2).
184. For a tungsten filament at a temperature of T \u003d 3500 K, the absorption capacity AT \u003d 0.35. Determine the radiation temperature of the thread. (Answer: 2.69 kK).
The spectral density of blackbody radiation is a universal function of wavelength and temperature. This means that the spectral composition and radiation energy of a black body do not depend on the nature of the body.
Formulas (1.1) and (1.2) show that knowing the spectral and integral radiation densities of an absolutely black body, one can calculate them for any non-black body if the absorption coefficient of the latter is known, which must be determined experimentally.
Research has led to the following laws of black body radiation.
1. Stefan-Boltzmann law: The integral radiation density of a blackbody is proportional to the fourth power of its absolute temperature
Value σ called Stephen's constant- Boltzmann:
σ \u003d 5.6687 10 -8 J m - 2 s - 1 K - 4.
Energy emitted over time t absolutely black body with a radiating surface S at constant temperature T,
W=σT 4 St
If the body temperature changes with time, i.e. T = T(t), then
The Stefan-Boltzmann law indicates an extremely rapid increase in radiation power with increasing temperature. For example, when the temperature rises from 800 to 2400 K (that is, from 527 to 2127 ° C), the radiation of a completely black body increases by 81 times. If a black body is surrounded by a medium with temperature T 0, then the eye will absorb the energy emitted by the medium itself.
In this case, the difference between the power of the emitted and absorbed radiation can be approximately expressed by the formula
U=σ(T 4 - T 0 4)
The Stefan-Boltzmann law is not applicable to real bodies, as observations show a more complex dependence R on temperature, and also on the shape of the body and the state of its surface.
2. Wien's displacement law. Wavelength λ 0, which accounts for the maximum spectral density of blackbody radiation, is inversely proportional to the absolute temperature of the body:
λ 0 = or λ 0 T \u003d b.
Constant b, called Wien's law constant, is equal to b= 0.0028978 m K ( λ expressed in meters).
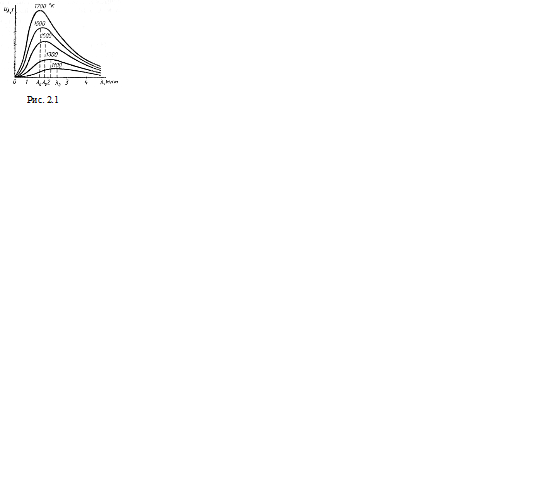
Thus, as the temperature rises, not only does the total radiation increase, but, in addition, the energy distribution over the spectrum changes. For example, at low body temperatures, infrared rays are mainly studied, and as the temperature rises, the radiation becomes reddish, orange, and finally white. On fig. Figure 2.1 shows the empirical distribution curves of blackbody radiation energy over wavelengths at different temperatures: it can be seen from them that the maximum spectral density of radiation shifts towards short waves with increasing temperature.
3. Planck's law. The Stefan-Boltzmann law and the Wien displacement law do not solve the main problem of how large is the spectral density of radiation per each wavelength in the spectrum of a black body at temperature T. To do this, you need to establish a functional dependency and from λ and T.
Based on the concept of the continuous nature of the emission of electromagnetic waves and on the law of uniform distribution of energy over degrees of freedom (accepted in classical physics), two formulas were obtained for the spectral density and radiation of a black body:
1) Win's formula
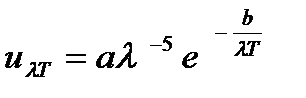
where a and b- constant values;
2) Rayleigh-Jeans formula
u λT = 8πkT λ – 4 ,
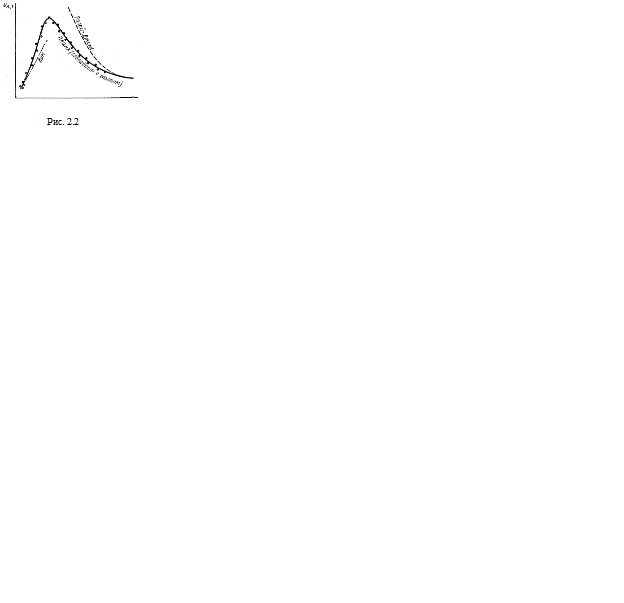 where k is the Boltzmann constant. Experimental verification showed that for a given temperature, Wien's formula is correct for short waves (when λT very small and gives sharp convergence of experience in the region of long waves. The Rayleigh-Jeans formula turned out to be correct for long waves and completely inapplicable for short ones (Fig. 2.2).
where k is the Boltzmann constant. Experimental verification showed that for a given temperature, Wien's formula is correct for short waves (when λT very small and gives sharp convergence of experience in the region of long waves. The Rayleigh-Jeans formula turned out to be correct for long waves and completely inapplicable for short ones (Fig. 2.2).
Thus, classical physics turned out to be unable to explain the law of energy distribution in the radiation spectrum of a completely black body.
To determine the type of function u λT completely new ideas about the mechanism of light emission were needed. In 1900, M. Planck hypothesized that absorption and emission of electromagnetic radiation energy by atoms and molecules is possible only in separate "portions", which are called energy quanta. The value of the quantum of energy ε proportional to the radiation frequency v(inversely proportional to the wavelength λ ):
ε = hv = hc/λ
Proportionality factor h = 6.625 10 -34 J s and is called Planck's constant. In the visible part of the spectrum for the wavelength λ = 0.5 μm, the value of the energy quantum is:
ε = hc/λ= 3.79 10 -19 J s = 2.4 eV
Based on this assumption, Planck obtained a formula for u λT:
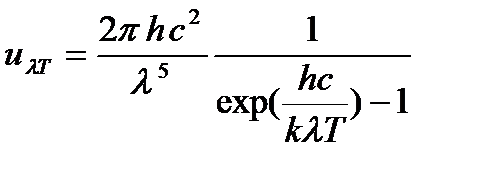 (2.1)
(2.1)
where k is the Boltzmann constant, With is the speed of light in vacuum. l The curve corresponding to function (2.1) is also shown in Fig. 2.2.
Planck's law (2.11) yields the Stefan-Boltzmann law and Wien's displacement law. Indeed, for the integral radiation density we obtain
Calculation according to this formula gives a result that coincides with the empirical value of the Stefan-Boltzmann constant.
Wien's displacement law and its constant can be obtained from Planck's formula by finding the maximum of the function u λT, for which the derivative of u λT on λ , and is equal to zero. The calculation results in the formula:
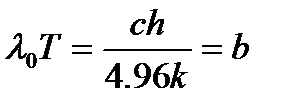 (2.2)
(2.2)
Calculation of the constant b according to this formula also gives a result coinciding with the empirical value of Wien's constant.
Let us consider the most important applications of the laws of thermal radiation.
 BUT. Thermal light sources. Most artificial light sources are thermal emitters (electric incandescent lamps, conventional arc lamps, etc.). However, these light sources are not economical enough.
BUT. Thermal light sources. Most artificial light sources are thermal emitters (electric incandescent lamps, conventional arc lamps, etc.). However, these light sources are not economical enough.
In § 1 it was said that the eye is sensitive only to a very narrow part of the spectrum (from 380 to 770 nm); all other waves have no visual sensation. The maximum sensitivity of the eye corresponds to the wavelength λ = 0.555 µm. Proceeding from this property of the eye, one should demand from light sources such a distribution of energy in the spectrum, in which the maximum spectral density of radiation would fall on the wavelength λ = 0.555 µm or so. If we take an absolutely black body as such a source, then according to Wien's displacement law, we can calculate its absolute temperature:
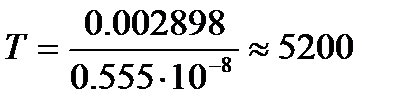 To
To
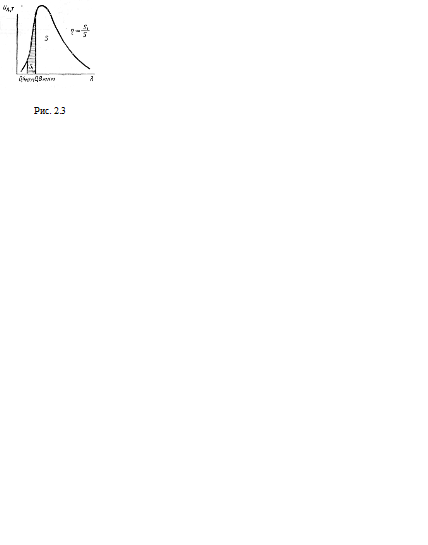
Thus, the most advantageous thermal light source should have a temperature of 5200 K, which corresponds to the temperature of the solar surface. This coincidence is the result of the biological adaptation of human vision to the distribution of energy in the spectrum of solar radiation. But even this light source efficiency(the ratio of the energy of visible radiation to the total energy of all radiation) will be small. Graphically in fig. 2.3 this coefficient is expressed by the ratio of areas S1 and S; square S1 expresses the radiation energy of the visible region of the spectrum, S- all radiation energy.
The calculation shows that at a temperature of about 5000-6000 K, the light efficiency is only 14-15% (for a completely black body). At the temperature of existing artificial light sources (3000 K), this efficiency is only about 1-3%. Such a low "light output" of a thermal emitter is explained by the fact that during the chaotic movement of atoms and molecules, not only light (visible), but also other electromagnetic waves are excited, which do not have a light effect on the eye. Therefore, it is impossible to selectively force the body to radiate only those waves to which the eye is sensitive: invisible waves are necessarily radiated.
The most important modern temperature light sources are electric incandescent lamps with a tungsten filament. The melting point of tungsten is 3655 K. However, heating the filament to temperatures above 2500 K is dangerous, since tungsten is very quickly sprayed at this temperature, and the filament is destroyed. To reduce filament sputtering, it was proposed to fill lamps with inert gases (argon, xenon, nitrogen) at a pressure of about 0.5 atm. This made it possible to raise the temperature of the filament to 3000-3200 K. At these temperatures, the maximum spectral density of radiation lies in the region of infrared waves (about 1.1 microns), so all modern incandescent lamps have an efficiency of slightly more than 1%.
B. Optical pyrometry. The above laws of radiation of a black body make it possible to determine the temperature of this body if the wavelength is known λ 0 corresponding to the maximum u λT(according to Wien's law), or if the value of the integral radiation density is known (according to the Stefan-Boltzmann law). These methods for determining body temperature by its thermal radiation on the cabins I optical pyrometry; they are especially convenient when measuring very high temperatures. Since the mentioned laws are applicable only to a completely black body, optical pyrometry based on them gives good results only when measuring the temperatures of bodies that are close in their properties to a completely black body. In practice, these are factory furnaces, laboratory muffle furnaces, boiler furnaces, etc. Consider three methods for determining the temperature of heat emitters:
a. Method based on Wien's displacement law. If we know the wavelength at which the maximum spectral density of radiation falls, then the temperature of the body can be calculated using formula (2.2).
In particular, the temperature on the surface of the Sun, stars, etc. is determined in this way.
For non-black bodies, this method does not give the true body temperature; if there is one maximum in the emission spectrum and we calculate T according to formula (2.2), then the calculation gives us the temperature of a completely black body, which has almost the same energy distribution in the spectrum as the body under test. In this case, the chromaticity of the radiation of a completely black body will be the same as the chromaticity of the radiation under study. This body temperature is called color temperature.
The color temperature of the filament of an incandescent lamp is 2700-3000 K, which is very close to its true temperature.
b. Radiation temperature measurement method based on the measurement of the integral radiation density of the body R and calculation of its temperature according to the Stefan-Boltzmann law. Appropriate instruments are called radiation pyrometers.
Naturally, if the radiating body is not absolutely black, then the radiation pyrometer will not give the true temperature of the body, but will show the temperature of an absolutely black body at which the integral radiation density of the latter is equal to the integral radiation density of the test body. This body temperature is called radiation, or energy, temperature.
Among the shortcomings of the radiation pyrometer, we point out the impossibility of using it to determine the temperatures of small objects, as well as the influence of the medium located between the object and the pyrometer, which absorbs part of the radiation.
in. I brightness method for determining temperatures. Its principle of operation is based on a visual comparison of the brightness of the incandescent filament of the pyrometer lamp with the brightness of the image of the incandescent test body. The device is a spotting scope with an electric lamp placed inside, powered by a battery. The equality visually observed through a monochromatic filter is determined by the disappearance of the image of the thread against the background of the image of a hot body. The glow of the thread is regulated by a rheostat, and the temperature is determined by the scale of the ammeter, graduated directly to the temperature.
photoelectric effect
 The photoelectric effect was discovered in 1887 by the German physicist G. Hertz and experimentally studied by A. G. Stoletov in 1888–1890. The most complete study of the phenomenon of the photoelectric effect was carried out by F. Lenard in 1900. By this time, the electron had already been discovered (1897, J. Thomson), and it became clear that the photoelectric effect (or, more precisely, the external photoelectric effect) consists in pulling electrons out of matter under the influence of light falling on it.
The photoelectric effect was discovered in 1887 by the German physicist G. Hertz and experimentally studied by A. G. Stoletov in 1888–1890. The most complete study of the phenomenon of the photoelectric effect was carried out by F. Lenard in 1900. By this time, the electron had already been discovered (1897, J. Thomson), and it became clear that the photoelectric effect (or, more precisely, the external photoelectric effect) consists in pulling electrons out of matter under the influence of light falling on it.
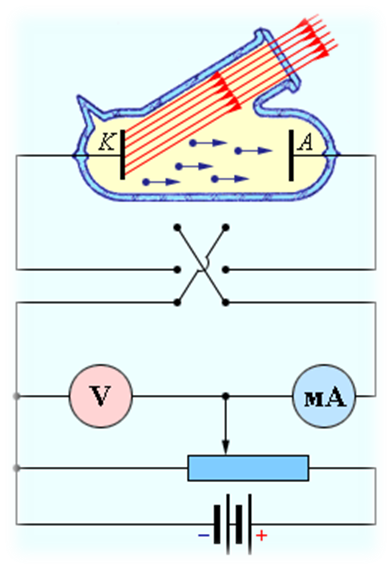
The layout of the experimental setup for studying the photoelectric effect is shown in fig. one.
| Rice. one |
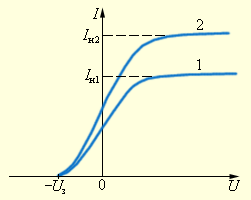 cleared. A voltage was applied to the electrodes U, the polarity of which could be changed using a double key. One of the electrodes (cathode K) was illuminated through a quartz window with monochromatic light of a certain wavelength λ. At a constant luminous flux, the dependence of the photocurrent strength was taken I from the applied voltage. On fig. Figure 2 shows typical curves of such a dependence, obtained for two values of the intensity of the light flux incident on the cathode.
cleared. A voltage was applied to the electrodes U, the polarity of which could be changed using a double key. One of the electrodes (cathode K) was illuminated through a quartz window with monochromatic light of a certain wavelength λ. At a constant luminous flux, the dependence of the photocurrent strength was taken I from the applied voltage. On fig. Figure 2 shows typical curves of such a dependence, obtained for two values of the intensity of the light flux incident on the cathode. The curves show that at sufficiently high positive voltages at the anode A, the photocurrent reaches saturation, since all the electrons ejected by light from the cathode reach the anode. Careful measurements have shown that the saturation current I n is directly proportional to the intensity of the incident light. When the voltage across the anode is negative, the electric field between the cathode and anode slows down the electrons. The anode can only reach those electrons whose kinetic energy exceeds | EU|. If the anode voltage is less than - U h, the photocurrent stops. measuring U h, it is possible to determine the maximum kinetic energy of photoelectrons: ( mυ 2 / 2)max = EU h
| Rice. one |
Numerous experimenters have established the following basic laws of the photoelectric effect:
1. The maximum kinetic energy of photoelectrons increases linearly with increasing light frequency ν and does not depend on its intensity.
2. For each substance there is the so-called red border of the photoelectric effect, i.e. the lowest frequency νmin at which the external photoelectric effect is still possible.
3. The number of photoelectrons pulled out by light from the cathode in 1 s is directly proportional to the light intensity.
4. The photoelectric effect is practically inertialess, the photocurrent occurs instantly after the start of cathode illumination, provided that the light frequency ν > ν min.
All these laws of the photoelectric effect fundamentally contradicted the ideas of classical physics about the interaction of light with matter. According to wave concepts, when interacting with an electromagnetic light wave, an electron would have to gradually accumulate energy, and it would take a considerable time, depending on the intensity of light, for the electron to accumulate enough energy to fly out of the cathode. Calculations show that this time should have been calculated in minutes or hours. However, experience shows that photoelectrons appear immediately after the start of illumination of the cathode. In this model, it was also impossible to understand the existence of the red boundary of the photoelectric effect. The wave theory of light could not explain the independence of the energy of photoelectrons from the intensity of the light flux and the proportionality of the maximum kinetic energy to the frequency of light.
Thus, the electromagnetic theory of light proved unable to explain these regularities.
A way out was found by A. Einstein in 1905. A theoretical explanation of the observed laws of the photoelectric effect was given by Einstein on the basis of M. Planck's hypothesis that light is emitted and absorbed in certain portions, and the energy of each such portion is determined by the formula E = h v, where h is Planck's constant. Einstein took the next step in the development of quantum concepts. He came to the conclusion that light has a discontinuous (discrete) structure. An electromagnetic wave consists of separate portions - quanta, subsequently named photons. When interacting with matter, a photon transfers all of its energy hν to one electron. Part of this energy can be dissipated by an electron in collisions with atoms of matter. In addition, part of the electron energy is spent on overcoming the potential barrier at the metal–vacuum interface. To do this, the electron must do the work function A out depending on the properties of the cathode material. The maximum kinetic energy that a photoelectron emitted from the cathode can have is determined by the energy conservation law:
![]()
This formula is called the Einstein equation for the photoelectric effect.
Using the Einstein equation, one can explain all the regularities of the external photoelectric effect. From the Einstein equation, the linear dependence of the maximum kinetic energy on frequency and independence on light intensity, the existence of a red border, and the inertia of the photoelectric effect follow. The total number of photoelectrons leaving the cathode surface in 1 s should be proportional to the number of photons falling on the surface in the same time. It follows from this that the saturation current must be directly proportional to the intensity of the light flux. This statement is called Stoletov's law.
As follows from the Einstein equation, the slope of the straight line expressing the dependence of the blocking potential U h on the frequency ν (Fig. 3), is equal to the ratio of the Planck constant h to the charge of an electron e:
This makes it possible to experimentally determine the value of Planck's constant. Such measurements were made in 1914 by R. Millikan and gave good agreement with the value found by Planck. These measurements also made it possible to determine the work function A:
![]()
where c is the speed of light, λcr is the wavelength corresponding to the red border of the photoelectric effect.
For most metals, the work function A is a few electron volts (1 eV = 1.602 10 -19 J). In quantum physics, the electron volt is often used as a unit of energy. The value of Planck's constant, expressed in electron volts per second, is h\u003d 4.136 10 -15 eV s.
Among metals, alkaline elements have the lowest work function. For example, sodium A= 1.9 eV, which corresponds to the red border of the photoelectric effect λcr ≈ 680 nm. Therefore, alkali metal compounds are used to create cathodes in photocells designed to detect visible light.
So, the laws of the photoelectric effect indicate that light, when emitted and absorbed, behaves like a stream of particles called photons or light quanta.
Thus, the doctrine of light, having completed a revolution lasting two centuries, again returned to the ideas of light particles - corpuscles.
But this was not a mechanical return to Newton's corpuscular theory. At the beginning of the 20th century, it became clear that light has a dual nature. When light propagates, its wave properties appear (interference, diffraction, polarization), and when interacting with matter, corpuscular (photoelectric effect). This dual nature of light is called wave-particle duality. Later, the dual nature was discovered in electrons and other elementary particles. Classical physics cannot give a visual model of the combination of wave and corpuscular properties of micro-objects. The motion of micro-objects is controlled not by the laws of classical Newtonian mechanics, but by the laws quantum mechanics. The theory of radiation of a completely black body, developed by M. Planck, and quantum theory Einstein's photoelectric effect is at the heart of this modern science.
In addition to the external photoelectric effect we have considered (usually called simply the photoelectric effect), there is also an internal photoelectric effect observed in dielectrics and semiconductors. It consists in the redistribution of electrons due to the action of light energy levels. In this case, electrons are released in the entire volume.
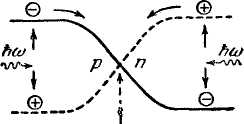 The action of the so-called photoresistors is based on the internal photoelectric effect. The number of current carriers formed is proportional to the incident light flux. Therefore, photoresistors are used for the purposes of photometry. Selenium was the first semiconductor to be used for this purpose.
The action of the so-called photoresistors is based on the internal photoelectric effect. The number of current carriers formed is proportional to the incident light flux. Therefore, photoresistors are used for the purposes of photometry. Selenium was the first semiconductor to be used for this purpose.
| Rice. 2 |
In the area of district transition or on the verge of a metal with a semiconductor, a gate photoelectric effect can be observed. It consists in the occurrence of an electromotive force (photo-emf) under the action of light. On fig. 173 shows the course of the potential energy of electrons (solid curve) and holes (dashed curve) in the region district transition. Minor carriers for this region (electrons in R-areas and holes in n-regions) that have arisen under the action of light pass through the transition. As a result, in p-region accumulates an excess positive charge, in n-regions - excess negative charge. This leads to the appearance of a voltage applied to the junction, which is the photoelectromotive force. In particular, this effect is used in the creation of solar panels.
