In what units is heat capacity measured? Heat capacity of gases. Types of heat capacities
The heat capacity of a body is the amount of heat that must be imparted to a given body in order to raise its temperature by one degree. When cooled by one degree, the body gives off the same amount of heat. The heat capacity is proportional to the mass of the body. The heat capacity of a unit mass of a body is called specific, and the product of specific heat by atomic or molecular mass is called atomic or molar, respectively.
Heat capacities various substances differ greatly from each other. So, specific heat water at 20 ° C is 4200 J / kg K, pine wood - 1700, air - 1010. For metals, it is less: aluminum - 880 J / kg K, iron - 460, copper - 385, lead - 130. Specific heat increases slightly with temperature (at 90°C, the heat capacity of water is 4220 J/kg K) and varies greatly during phase transformations: the heat capacity of ice at 0°C is 2 times less than that of water; the heat capacity of water vapor at 100°C is about 1500 J/kg K.
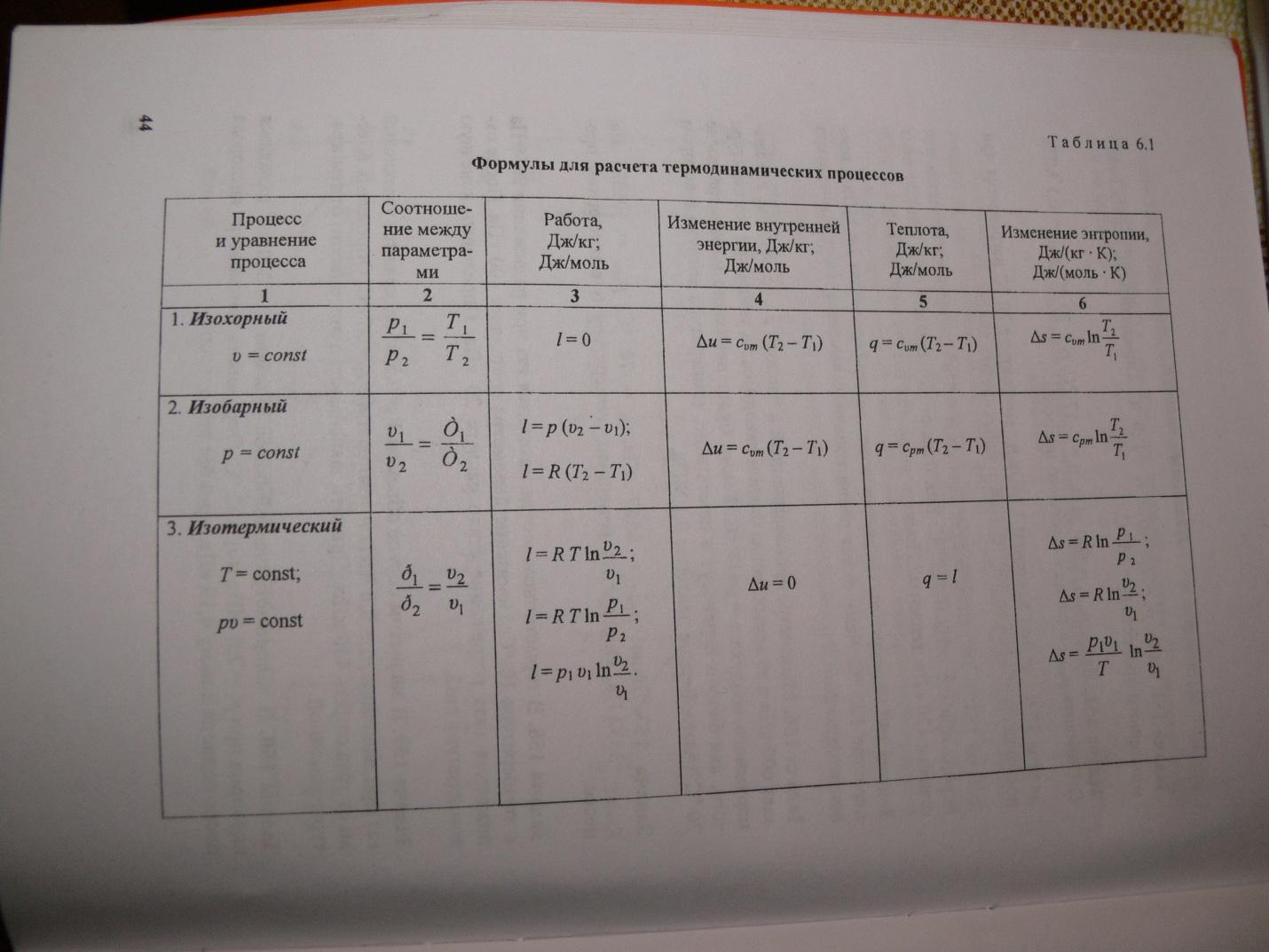
The heat capacity depends on the conditions in which the temperature of the body changes. If the dimensions of the body do not change, then all the heat goes to change the internal energy. Here we are talking about heat capacity at constant volume. At constant external pressure, due to thermal expansion, mechanical work is performed against external forces, and heating to a particular temperature requires more heat. Therefore, the heat capacity at constant pressure is always greater than . For ideal gases(see figure), where R is the gas constant, equal to 8.32 J / mol K.
Usually measured. Classic way heat capacity measurements are as follows: a body whose heat capacity is to be measured is heated to a certain temperature and placed in a calorimeter with an initial temperature of , filled with water or another liquid with a known heat capacity and - the heat capacity of the calorimeter and liquid).
By measuring the temperature in the calorimeter after thermal equilibrium has been established, the heat capacity of the body can be calculated using the formula:
![]()
where and are the masses of the body, liquid, and calorimeter.
The most developed theory is the heat capacity of gases. At ordinary temperatures, heating leads mainly to a change in the energy of the translational and rotational motion of gas molecules. For the molar heat capacity of monatomic gases, the theory gives , diatomic and polyatomic - and . At very low temperatures, the heat capacity is somewhat less due to quantum effects (see Fig. Quantum mechanics). At high temperatures vibrational energy is added, and the heat capacity of polyatomic gases increases with increasing temperature.
The atomic heat capacity of crystals, according to the classical theory, is equal to , which is consistent with the empirical law of Dulong and Petit (established in 1819 by French scientists P. Dulong and A. Petit). Quantum theory heat capacity leads to the same conclusion at high temperatures, but predicts a decrease in heat capacity with decreasing temperature. Near absolute zero, the heat capacity of all bodies tends to zero (the third law of thermodynamics).
Heat capacity of the body- this is physical quantity, determined by the ratio of the amount of heat absorbed by the body when heated, to the change in its temperature:
The physical meaning of the heat capacity of a body: the heat capacity of a body is equal to the amount of heat absorbed by the body when heated or released when it is cooled by 1K.
Since the heat capacities are variable, they distinguish between the average and true heat capacities. Under the average heat capacity understand the ratio of the amount of heat q , summed up to a unit of the amount of a substance (gas), to a change in its temperature from t 1 before t 2 provided that the temperature difference t 2 – t 1 is a finite value. The average mass, volume, and molar heat capacities, respectively, are denoted by c m , c m ' and m . From the definition of the average heat capacity it follows that if the gas temperature rises from t 1 before t 2 then its average heat capacity [kJ / (kg * K)]
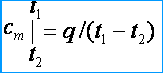
Under the true heat capacity understand the heat capacity of the gas, corresponding to an infinitesimal change in gas temperature, corresponding to an infinitesimal change in temperature dt , i.e.
c = dq/dt,
where dq=cdt.
Specific heat is the ability of different substances to absorb heat when they are heated. The specific heat capacity of a substance is determined by the ratio of the amount of heat received by it when heated to the mass of the substance and the change in its temperature, if: ![]()
the relationship expressing the relationship between the molar heat capacities Cp and CV has the form (Mayer's formula): Cp = CV + R. OR MORE EXPANDED Heat capacity ideal gas If, as a result of heat transfer, a certain amount of heat is transferred to the body, then the internal energy of the body and its temperature change. The amount of heat Q required to heat 1 kg of a substance by 1 K is called the specific heat of the substance c. c = Q / (mΔT). In many cases, it is convenient to use the molar heat capacity C: C = M c, where M is the molar mass of the substance. The heat capacity determined in this way is not an unambiguous characteristic of a substance. According to the first law of thermodynamics, the change in the internal energy of a body depends not only on the amount of heat received, but also on the work done by the body. Depending on the conditions under which the heat transfer process was carried out, the body could perform various work. Therefore, the same amount of heat transferred to the body could cause different changes in its internal energy and, consequently, temperature. Such ambiguity in determining the heat capacity is typical only for a gaseous substance. When liquid and solid bodies are heated, their volume practically does not change, and the work of expansion turns out to be equal to zero. Therefore, the entire amount of heat received by the body goes to change its internal energy. Unlike liquids and solids, the gas in the process of heat transfer can greatly change its volume and do work. Therefore, the heat capacity of a gaseous substance depends on the nature of the thermodynamic process. Usually, two values of the heat capacity of gases are considered: CV is the molar heat capacity in an isochoric process (V = const) and Cp is the molar heat capacity in an isobaric process (p = const). In the process at a constant volume, the gas does no work: A = 0. From the first law of thermodynamics for 1 mole of gas follows QV = CVΔT = ΔU. The change ΔU of the internal energy of a gas is directly proportional to the change ΔT of its temperature. For a process at constant pressure, the first law of thermodynamics gives: Qp = ΔU + p(V2 - V1) = CVΔT + pΔV, where ΔV is the change in volume of 1 mole of an ideal gas when its temperature changes by ΔT. It follows from this: The ratio ΔV / ΔT can be found from the equation of state of an ideal gas written for 1 mole: pV = RT, where R is the universal gas constant. At p = const Thus, the relation expressing the relationship between the molar heat capacities Cp and CV has the form (Mayer's formula): Cp = CV + R.
The gas constant is numerically equal to the work of expansion of 1 mole of an ideal gas under constant pressure when heated by 1 K. R = pV/T = 1.01 10 5 22.4 10-3/273[Pa m 3 /mol]/K =8.31(44) Dzh/ (mol K)
The universal gas constant is a universal, fundamental physical constant R, equal to the product of the Boltzmann constant k and the Avogadro constant
Physical meaning: Gas constant i is numerically equal to the work of expansion of one mole of an ideal gas in an isobaric process with an increase in temperature by 1 K
In the CGS system, the gas constant is:
The specific gas constant is:
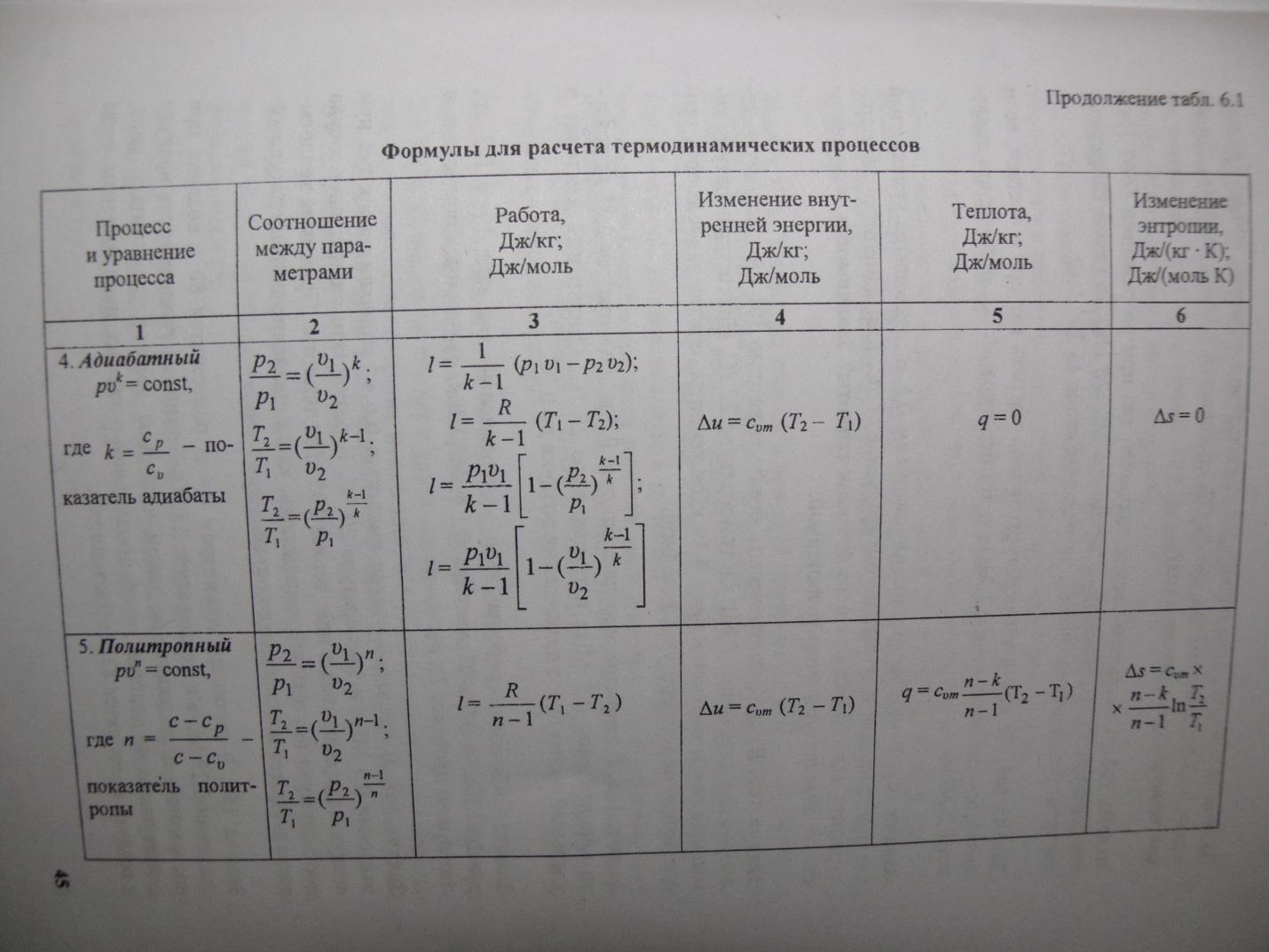
Adiabatic exponent(sometimes called coefficientPoisson) - the ratio of heat capacity at constant pressure () to heat capacity at constant volume (). Sometimes it is also called factor isentropic extensions. Denoted by the Greek letter (gamma) or (kappa). The letter symbol is mainly used in chemical engineering disciplines. In heat engineering, the Latin letter is used.
A mixture of gases is a collection of several dissimilar gases that, under the conditions under consideration, do not enter into chemical reactions with each other.
A mixture of gases is a homogeneous thermodynamic system (inside which there are no interfaces separating macroscopic parts of the system from each other, differing in their properties and composition).
Partial pressure P i of the i-th gas in the mixture is the pressure under which this gas would be if all other gases were removed from the mixture, and V and T remained the same.
Dalton's law - The pressure of a mixture of gases that do not chemically interact with each other is equal to the sum of the partial pressures of these gases.
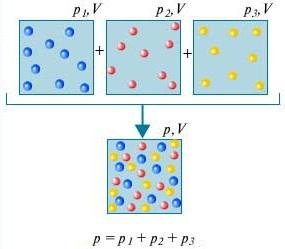
In order to understand what is dalton's law, consider for this the air in the room. It is a mixture of several gases: nitrogen (80%), oxygen (20%). The partial pressure of each of these gases is the pressure that the gas would have if it alone occupied the entire volume. For example, if all gases except nitrogen were removed from the room, then the pressure of what was left would be the partial pressure of nitrogen. Dalton's law states that the total pressure of all gases taken together is equal to the sum of the partial pressures of each gas taken separately. (Strictly speaking, the law applies only to ideal gases, but to a good enough approximation it also describes real gases.)
First law of thermodynamics is a generalization of the law of conservation and transformation of energy for a thermodynamic system. It is formulated as follows:
Change ΔU internal energy of a non-isolated thermodynamic system is equal to the difference between the amount of heatQ transferred to the system, and workA , a perfect system over external bodies.
|
The relation expressing the first law of thermodynamics is often written in a different form:
|
The amount of heat received by the system is used to change its internal energy and perform work on external bodies.
The first law of thermodynamics is a generalization of experimental facts. According to this law, energy cannot be created or destroyed; it is transferred from one system to another and is transformed from one form into another. An important consequence of the first law of thermodynamics is the assertion that it is impossible to create a machine capable of doing useful work without consuming energy from the outside and without any changes inside the machine itself. Such a hypothetical machine is called perpetual motion machine (perpetuum mobile) of the first kind . Numerous attempts to create such a machine invariably ended in failure. Any machine can do positive work A over external bodies only by obtaining a certain amount of heat Q from surrounding bodies or a decrease in Δ U its internal energy.
Let us apply the first law of thermodynamics to isoprocesses in gases.
With isobaric expansion Q> 0 - heat is absorbed by the gas, and the gas does positive work. With isobaric compression Q < 0 – тепло отдается внешним телам. В этом случае A < 0. Температура газа при изобарном сжатии уменьшается, T 2 < T one ; internal energy decreases, Δ U < 0.
AT isothermal process the gas temperature does not change, therefore, the internal energy of the gas does not change, Δ U = 0.
AT isochoric process (V= const) the gas does no work, A= 0. Therefore,
The first law of thermodynamics for an isobaric process gives:
|
The first law of thermodynamics for an isothermal process is expressed by the relation
|
Quantity of heat Q, obtained by the gas in the process of isothermal expansion, turns into work on external bodies. Under isothermal compression, the work of external forces produced on the gas is converted into heat, which is transferred to the surrounding bodies.
Along with isochoric, isobaric, and isothermal processes, thermodynamics often considers processes that occur in the absence of heat exchange with surrounding bodies. Vessels with insulating walls are called adiabatic shells, and the processes of gas expansion or compression in such vessels are called adiabatic.
AT adiabatic processQ= 0; so the first law of thermodynamics takes the form
|
|
|
In its physical meaning, the first law of thermodynamics is the law of conservation (change) of energy in thermodynamics. If, according to the law of energy change in mechanics, the work of non-conservative forces is equal to the increment of the mechanical energy of the system (in particular, the work of friction forces having a negative sign is equal to the decrease in the mechanical energy of the system), then according to the first law of thermodynamics, the increment of the internal energy of the thermodynamic system is equal to the sum of the work of external forces , perfect over the system, and the energy transferred to the system by heat transfer. Enthalpy(from Greek. enthalpo- heat up) is property of matter, indicating the amount of energy that can be converted into heat. Enthalpy is a thermodynamic property of a substance that indicates the level of energy stored in its molecular structure. This means that while matter can have energy based on temperature and pressure, not all of it can be converted to heat. Part of the internal energy always remains in the substance and maintains its molecular structure. Part of the kinetic energy of a substance is not available when its temperature approaches the ambient temperature. Consequently, enthalpy is the amount of energy that is available for conversion into heat at a given temperature and pressure. The units of enthalpy are BTU or Joule for energy and Btu/lbm or J/kg for specific energy. 11 question |
Objective: Experimental determination of the heat capacity of materials.
Equipment: Scales, weight, calorimeter, metal cylinder with known heat capacity, beaker, heater, hook.
Brief theory:
All macroscopic bodies and systems of bodies, in addition to mechanical energy due to their movement and interaction, have energy that depends on their internal state. This energy, which is the energy of movement and interaction between all the particles that make up the body, is called internal.
Internal energy includes the kinetic energy of the thermal motion of molecules and atoms that make up a given body, the kinetic energy of electrons moving in atoms around nuclei, the potential energy of interactions between molecules, atoms, electrons and nuclei, nucleons in the nucleus, etc. The concept of internal energy does not include the kinetic and potential energy of a given body as a whole.
The internal energy is uniquely determined by the set of parameters characterizing the state of the given system, i.e. is a single-valued function of the state of this system.
Internal energy is usually denoted by the letter U.
In thermal phenomena occurring at temperatures far from the temperatures of phase transitions, a change in the internal energy DU is associated with a change in the kinetic and potential energy of the molecules, while the remaining components of the internal energy do not change. Therefore, in such processes, we can assume that the internal energy of a body is equal to the sum of the kinetic energies of the chaotic thermal motion of all molecules relative to the center of mass of this body and the potential energies of interaction of all molecules with each other.
When the state of the body changes, its internal energy changes. For example, when the temperature of a body rises, its internal energy increases, since the average kinetic energy of the movement of the molecules of this body increases. As the temperature decreases, the internal energy of the body decreases.
The internal energy of bodies can change different ways. For example, internal energy changes when mechanical work is done by external forces on a given body during its deformation, and also without work being done, when the body is in contact with another body (or medium) that has a higher or lower temperature than the given body.
The process of changing the internal energy of a body without performing mechanical work is called heat transfer or heat transfer. There are three types of heat transfer: convection, conduction and radiation.
During heat exchange, there is no conversion of energy from one type to another. The process of heat transfer consists in the fact that part of the internal energy from a hotter body is transferred to a less hot body (or medium).
To characterize the processes of heat transfer, the concept of the amount of heat is introduced, which is called a quantity that is a quantitative measure of the change in the internal energy of the body in the process of heat transfer.
It must be remembered that the body can only give or receive energy, and the amount of heat Q is only a numerical equivalent of the energy given or received by the body in the process of heat transfer.
The amount of heat depends on the type of process and is not a function of the state of the system.
The amount of heat required to heat the body that occurs without phase transformations (without changing the state of aggregation of the substance):
where c is the specific heat capacity of the body, determined by the ratio of the amount of heat transferred to the mass m of the body, and the resulting temperature change DT, m is the body mass, DT is the difference between the final and initial temperatures of the given body.
The amount of heat that needs to be imparted to the body in order to raise its temperature by one Kelvin is called the heat capacity of this substance. When cooled by one Kelvin, the body gives off the same amount of heat. The heat capacity of a body is proportional to the mass of the body and depends on the substance of which it is composed. In the SI system of units, heat capacity is measured in J/K.
To characterize the thermal properties of a substance, the heat capacity of a unit mass of this substance is taken. This characteristic is called specific heat capacity. It is equal to the ratio of the heat capacity of a given body to its mass. The specific heat capacity with the SI system is measured in J / (kg × K).
Experimentally, the specific heat of a metal body is determined using a calorimeter and a thermometer. The simplest calorimeter consists of a polished metal glass placed inside another glass on stoppers (for the purpose of thermal insulation). The inner glass is filled with water or another liquid with a known specific heat capacity. A body heated to a certain temperature t is lowered into a calorimeter. Let the temperature of the liquid in the calorimeter be t 1 before the body is lowered, and after the thermal equilibrium of the liquid and the body lowered into it is established, their total temperature will become equal to q.
From the law of conservation of energy it follows that:
Q \u003d Q 1 + Q 2,(2)
where is the amount of heat Q given by a heated body is equal to the sum of the amount of heat Q1, received by water, and Q2, received by the calorimeter.
Considering (1), we rewrite (2) in the form:
cm (t-q) \u003d c 1 m 1 (q-t 1) + c 2 m 2 (q-t 1),(3)
where c 1 and m 1- specific heat capacity and mass of water in the calorimeter, c 2 and m2- specific heat capacity and mass of the calorimeter. This equation is called the heat balance equation. From it we find the specific heat capacity of the body:
It is possible to approach the solution of this problem in a different way, suppose that the energy losses in the framework of one experiment will be the same. Body With known specific heat capacity, heated to a certain temperature t, lowered into a calorimeter, the temperature of which changes. Then the heat balance equation will take the form:
cm(t-q) = c 1 m 1 (q-t 1) + Q loss,(5)
where Q loss is the amount of heat received by the calorimeter and environment, Consequently:
Q loss, = cm(t-q) - c 1 m 1 (q-t 1). (6)
If a body with an unknown specific heat capacity, heated to the same temperature, is lowered into a calorimeter, then the heat balance equation will take the form:
c x m x (t-q x) = c 1 m¢ 1 (q - t¢ 1) + Q losses,(7)
where m¢ 1 is the mass of water in the calorimeter, and t¢ 1- water temperature in this experiment. Then:
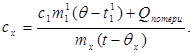 (8)
(8)
Substituting the value (6) into (8) we get:
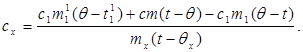 (9)
(9)
where is the elementary amount of heat; - an elementary change in temperature.
The heat capacity is numerically equal to the amount of heat that must be supplied to the system in order to increase its temperature by 1 degree under given conditions. Since the unit of heat in SI is the joule, and temperature is the degree K, the unit of heat capacity will be J / K.
Depending on the quantitative unit of the body to which heat is supplied in thermodynamics, mass, volume and molar heat capacities are distinguished.
Mass heat capacity is the heat capacity per unit mass of the working fluid,
where and are the volume and density of the body under normal physical conditions.
Volumetric heat capacity is measured in J/(m3 K).
Molar heat capacity- heat capacity, referred to the amount of the working fluid (gas) in moles,
| , |
where m3/mol is the molar volume of gas under normal conditions.
Considering that the heat capacity is not constant, but depends on temperature and other thermal parameters, a distinction is made between true and average heat capacity. Usually, the true heat capacity is understood as the ratio of the elementary amount of heat that is reported to the thermodynamic system in any process to the infinitely small increment in the temperature of this system, caused by the imparted heat. We will consider the true heat capacity of the thermodynamic system at a system temperature equal to , and - true specific heat capacity working fluid at its temperature equal to . Then the average specific heat capacity of the working fluid when its temperature changes from to can be determined as
Determination of the internal energy of the body.
Internal energy body (referred to as E or U) is the sum of the energies of molecular interactions and thermal motions of a molecule. In particular, the internal energy of an ideal gas is equal to the sum of the kinetic energies of all gas particles in continuous and random thermal motion. From this follows Joule's law, confirmed by numerous experiments.
The molecular kinetic theory leads to the following expression for the internal energy of one mole of an ideal monatomic gas (helium, neon, etc.), whose molecules perform only translational motion:
In this way, internal energy U body is uniquely determined by macroscopic parameters characterizing the state of the body. It does not depend on how the given state was realized. It is customary to say that internal energy is a state function.
In TD, the change in internal energy is used, and not its absolute value.
What is expansion work. The first law of TD.
Expansion work - the mechanical work performed by the vehicle against the forces of external pressure in the process of its expansion. When the volume changes, the gas does work, the sign of the change in V coincides with the sign of the work
The first law of TD: the heat imparted to the system is used to convert internal energy and do work.
From the point of view of its ability to receive (or give) energy in the form of heat, it is customary to characterize a thermodynamic system by its heat capacity.
The heat capacity of a body (system) is a physical quantity that is numerically equal to the amount of heat that must be imparted to the body (system) in order to change its temperature by one Kelvin.
If the body is given an infinitesimal amount of heat Q, which caused an infinitesimal increase in temperature dT, then its heat capacity FROM is by definition equal to
The SI unit of heat capacity is the joule divided by the kelvin ( J/To).
Experiments and theoretical calculations show that the heat capacity of a body depends on its chemical composition, mass and thermodynamic state(for example, on temperature), as well as on the type of process of changing the state of the body when heat is imparted to it.
The specific heat capacity is the heat capacity per unit mass of a substance, that is, for a homogeneous substance
![]() , (25.2)
, (25.2)
where With- specific heat capacity, M is the mass of the substance.
The SI unit of specific heat is the joule divided by a kelvin-kilogram [( J/(To . kg)].
The molar heat capacity is the heat capacity of one mole of a substance, that is
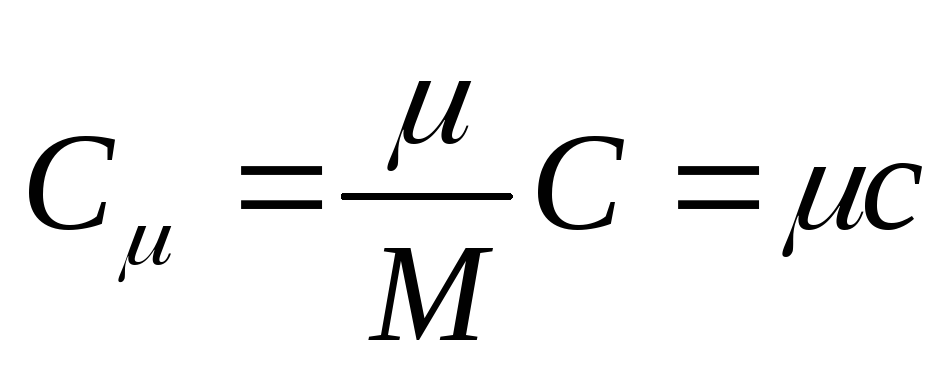 , (25.3)
, (25.3)
where FROM - molar heat capacity; is the molar mass of the substance.
The unit of molar heat capacity in SI is the joule divided by the kelvin-mole [ J/(To . mole)].
Elementary amount of heat Q, needed to change body temperature dT, is defined as
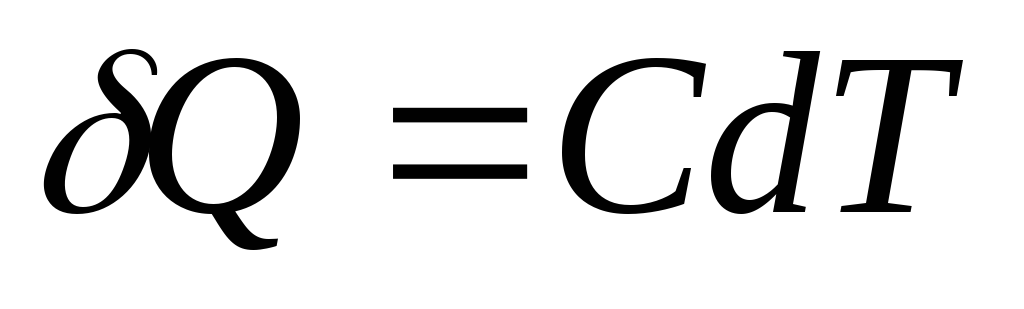 ,
,
and for a homogeneous body
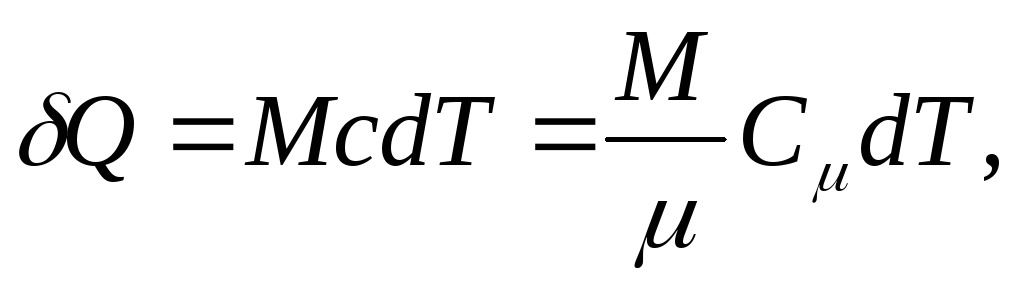 (25.4)
(25.4)
where M/ - amount of substance (number of moles).
§26. First law of thermodynamics
The first law (first law) of thermodynamics is a mathematical expression of the law of conservation and transformation of energy as applied to thermodynamic systems. It was established as a result of experimental and theoretical research in the field of physics and chemistry, the final stage of which was the discovery of the equivalence of heat and work, that is, the discovery that the conversion of heat into work and work into heat is always carried out in the same strictly constant quantitative ratio .
In §24 it was noted that the internal energy of a system can be changed in two ways: by doing work and by transferring heat. Therefore, we can write:
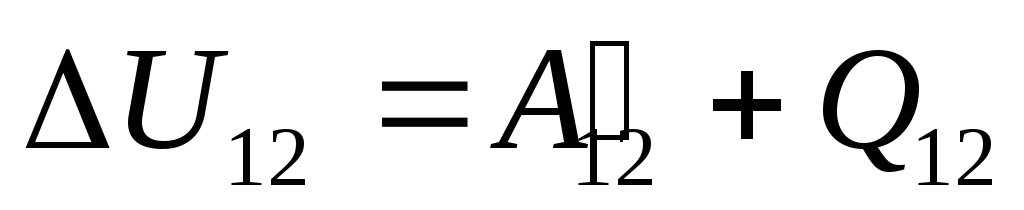 ,
(26.1)
,
(26.1)
where
U 12
-
change in the internal energy of the system during its transition from the state 1
into a state 2
as a result of work on it  from external bodies and transferring to it from the outside a certain amount of heat
from external bodies and transferring to it from the outside a certain amount of heat  .
.
We know that work  performed by the system itself on external bodies is numerically equal and opposite in sign to the work
performed by the system itself on external bodies is numerically equal and opposite in sign to the work 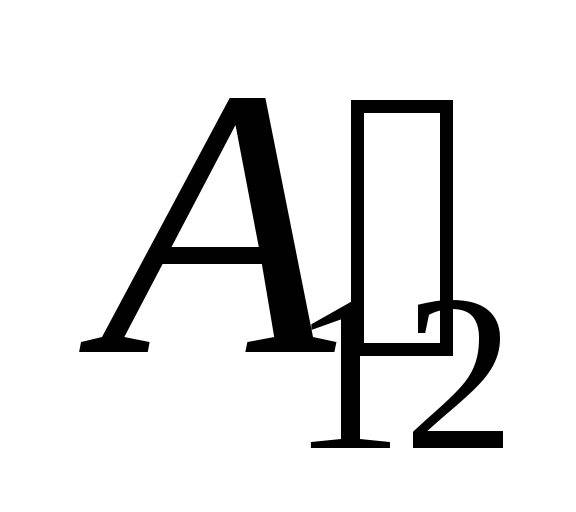 external bodies above the system, that is
external bodies above the system, that is
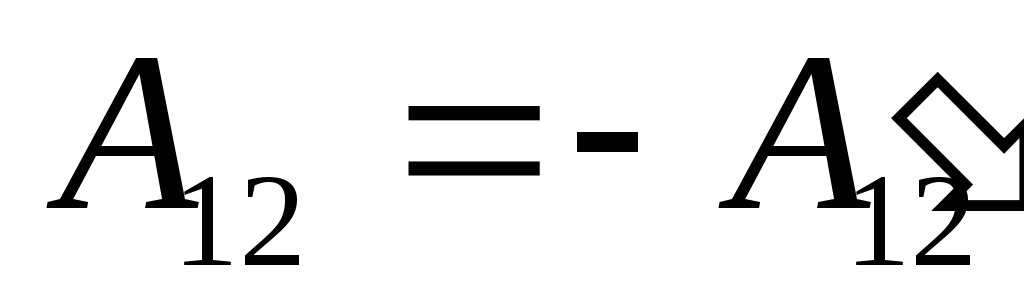 ,
(26.2)
,
(26.2)
therefore expression (26.1) can be rewritten as
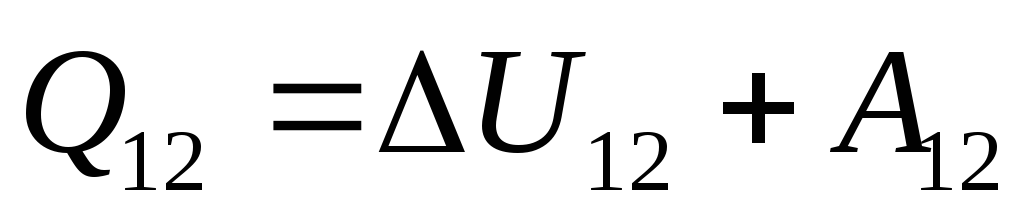 . (26.3)
. (26.3)
This equation is a mathematical record of the first law of thermodynamics: the amount of heat communicated to the system is spent on changing its internal energy and on performing work on external bodies by the system.
With an infinitesimal change in the state of the system, equation (26.3) takes the form
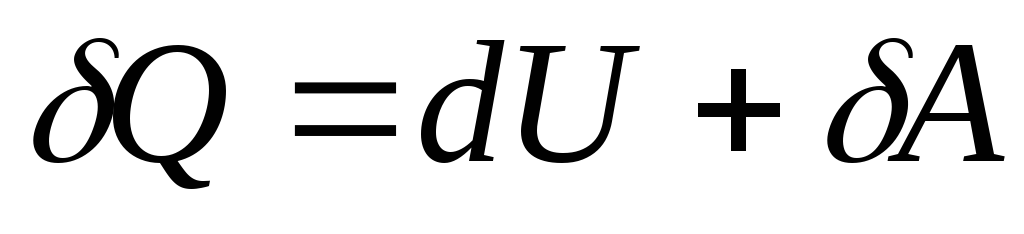 , (26.4)
, (26.4)
where 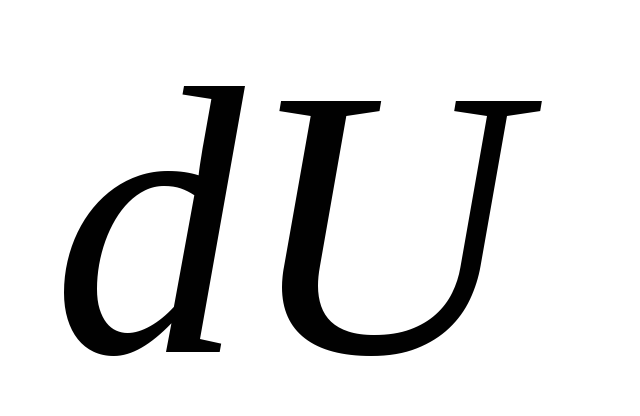 - an infinitesimal change in the internal energy of the system when an elementary amount of heat is imparted to it
Q and performing elementary work by the system
BUT over external bodies.
- an infinitesimal change in the internal energy of the system when an elementary amount of heat is imparted to it
Q and performing elementary work by the system
BUT over external bodies.
