How to determine the specific heat capacity of water. Big encyclopedia of oil and gas
INSTRUMENTS AND ACCESSORIES: calorimeter, thermometer, scales, test bodies, beaker (calibrated in grams), electric heater.
– experimentally confirm the validity of the heat balance equation;
– calculate the specific heat capacities of solids;
– arrange the results of measurements and calculations in the form of a table;
– write down your suggestions for improving the technique of measurements and calculations in this work.
A BRIEF THEORY OF EXPERIENCE
One of the basic physical concepts of thermodynamics is heat capacity.
Heat capacity of the body called physical quantity, numerically equal to heat, which must be communicated to the body in order to change its temperature by 1 K in the considered thermodynamic process. On the other hand, the heat capacity of a body is equal to the ratio of the heat dQ imparted to the body to the change dT in the body temperature in the thermodynamic process under consideration:
The heat capacity of a body depends on its chemical composition, mass of the body and its thermodynamic state, and also, as can be seen from the definition, on the type of process of changing the state of the body, in which heat dQ enters.
Thermal properties homogeneous bodies are characterized by the values of specific and molar (molar) heat capacity. The specific heat capacity of a substance called a physical quantity With, numerically equal to the heat that must be imparted to one kilogram of a substance to change its temperature by 1 K in the thermodynamic process under consideration. The heat capacity of a homogeneous body can be defined as the product of the mass of the body m for specific heat capacity With its substances:
![]() or (2.2).
or (2.2).
Thus, the relationship between dQ and dT for a homogeneous body has the form:
molar heat capacity the physical quantity C is called, numerically equal to the heat that must be imparted to one mole of a substance to change its temperature by 1 K in the thermodynamic process under consideration:
FROM = MS = (2.4),
where M is the molar mass of the substance; FROM is its specific heat capacity in the same process.
Expression (2.4) can now be written in the form:
where = n is the amount of substance.
The unit of measure for the heat capacity of a body is 1 J/K, specific heat– 1 J/kg. K, molar - 1 J / mol. TO.
If heating occurs under conditions where the volume remains constant, then the corresponding molar heat capacity is called heat capacity at constant volume, or isochoric heat capacity, and is denoted C v:
If the pressure remains constant during heating, then the heat capacity is called heat capacity at constant pressure C p (it can also be called isobaric heat capacity):
Note that for solids, only the heat capacity at constant pressure, and not at constant volume, is available for direct measurement, since due to thermal expansion it is impossible to ensure the constancy of the volume of the body. However, due to the smallness of the change in volume during heating, the difference between the heat capacities C p and C v is small.
Experimentally, the heat capacity of a body is determined by applying the heat balance equation. Let the body be heated to a temperature that is higher than the temperature environment. Then, cooling down, the body gives off a certain amount of heat. According to the law of conservation of energy in a closed system, the amount of heat received by the medium must be exactly equal to the amount of heat given off by the body. In this work, cooling down, the test body gives off heat to the water in the calorimeter and to the calorimeter itself.
Let the given test body with mass m, heated to a temperature t0, is lowered into the calorimeter with water, the temperature of which t1. As a result of heat transfer water temperature and the calorimeter rises to t2, a Body temperature drops to t2. The amount of heat given off by the body is:
Q dep = cm(t0 - t2) (2.6),
where c is the specific heat capacity of the test body,
t0– initial body temperature,
t2– end body temperature,
m- body mass.
The amount of heat received by the calorimeter and water is equal to:
where and are the mass and specific heat of the calorimeter,
I - mass and specific heat capacity of water,
t1– initial water temperature,
t2 is the final temperature of the water.
According to the law of conservation of energy in a closed system:
Q otd \u003d Q floor (2.8).
Then, substituting formulas 2.6 and 2.7 into equation 2.8 and expressing the desired value FROM , we get:
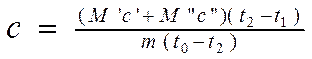 (2.9).
(2.9).
DETERMINE THE SPECIFIC HEAT CAPACITIES OF THREE CYLINDRICAL BODIES FROM VARIOUS METALS
1. Determine the values of the masses of bodies - m i, mass of the calorimeter – , specific heat of water – , specific heat of the calorimeter – .
2. Pour into the calorimeter a measured amount of cold water at room temperature (approximately 150 g).
3. Measure the initial temperature of cold water t 1 .
4. Heat the water in the vessel to a boil.
5. Place one of the test bodies in boiling water for a while. Take the temperature of the heated body t 0 equal to the temperature boiling water under normal conditions - 100 ° C.
6. Place the heated body in the calorimeter with water. Wait for the end of heat transfer and measure the final temperature in the calorimeter - t 2 .
8. Determine similarly the specific heat capacities of the other two bodies.
9. Record the results of measurements and calculations in table 3.
10. Based on the values of specific heat capacities, determine the substances from which the bodies are made.
12. Having measured the linear dimensions of the bodies, determine their density.
13. Calculate the errors and record the result in accordance with GOST.
14. Write down the conclusions on the laboratory work.
Table 3
| No. p / p | M", kg | M", kg | m, kg | C", J / kg. K | C", J / kg. K | t 0 , 0 C | t 1 , 0 C | t 2 , 0 C | C, J/kg. To |
Page 2
The accuracy of determining the specific heat according to Sykes is very high. However, this method is associated with greater experimental difficulties than the Smith method, and also gives accurate results only for heating curves, although it can also be used to obtain cooling curves. Smith's method makes it easier to study narrow temperature ranges, but is probably less accurate.
Therefore, in order to determine the specific heat capacity sg of a substance, it is necessary to measure the work A done by external forces acting on the body, and to measure the change in body temperature observed as a result of the work in the absence of heat exchange with other bodies.
The most commonly used method for determining the specific heat capacity; called mixing. The calorimeter (Regno) consists of a vessel of red copper, placed on wooden legs on the bottom of another copper vessel, from which it is separated by a layer of air, the thermal conductivity and heat capacity of which, per unit volume, are insignificant. The first vessel is filled with water.
Let us now consider methods for determining the specific heat capacity of a gas mixture.
The direct (direct) determination of the specific heat capacity Su and the study of the course of its change depending on temperature and specific volume is one of the effective means study of the critical state of substances. Therefore, experimental determinations of heat capacity are of great theoretical and practical interest in the study of critical phenomena.
What is the formula for determining the specific heat capacity of solutions.
When conducting experiments to determine the specific heat by mixing, it is necessary to heat the test sample - - to a precisely fixed temperature. For this, portable heaters are used, which are installed above the calorimeter for a short time, necessary for the rapid transfer of the heated sample to the calorimeter.
| Device diagram. |
The essence of the simplest absolute stationary method for determining the specific heat capacity is as follows: a sample of the material under test with a thickness h and a cross-sectional area of 5 is placed between the heater and the cooler. The heater can be a vessel with hot water or an electric heating element so that its power can be adjusted as desired by changing the voltage. The cooler is a hollow metal body through which cold water is passed. The temperatures on the heated and cooled surfaces of the sample (tj and, respectively, / 2) are measured by thermocouples.
Below is an example of processing experimental data when determining the specific heat capacity of a sandstone sample. A hollow cylinder filled with powder of the studied rock sample was heated to 40 C and cooled in a still air chamber to a temperature of 18–20 C.
On fig. 3 - 6 shows nomograms for determining the specific heat capacity of liquid individual hydrocarbons and oil mixtures, as well as aqueous solutions of methanol and ethanol.
For example, let's make a heat balance equation, which is used in determining the specific heat capacity of a substance using a calorimeter. Approximately, it can be considered that in this case three bodies participate in heat exchange: a calorimeter, a liquid and a body, the specific heat of the substance of which is determined.
6.4. Heat transfer between bodies
6.4.1. Heat capacity of the body, specific heat capacity of a substance, molar heat capacity of a substance
In order to raise the temperature of the body, it needs to communicate a certain amount of heat.
1 kg of a given substance per 1 K is called specific heat substances and is calculated by the formula
c beats = Q m Δ T ,
where Q is the amount of heat required to heat a certain mass of matter; m is the mass of the substance; ΔT is the change in the temperature of the substance when heated.
In the International System of Units, the specific heat capacity of a substance is measured in joules divided by kilogram-kelvin (1 J/(kg ⋅ K)).
The amount of heat required to heat some mass of matter, is determined by the product
Q = c beat m ∆T .
The amount of heat required to raise a given body by 1 K is called heat capacity of the body and is calculated by the formula
C = QΔT,
where Q is the amount of heat required to heat a given body; ΔT - change in body temperature when heated.
In the International System of Units, the heat capacity of a body is measured in joules divided by kelvin (1 J/K).
The amount of heat required to heat a certain body is determined by the product
Q=CΔT,
where C is the heat capacity of the body.
The heat capacity of a body and the heat capacity of the substance of which the body is composed, interconnected expression
C \u003d mc beats,
where C is the heat capacity of the body; m - body weight; c beat is the specific heat capacity of the substance from which this body is made.
The amount of heat required to raise the temperature of 1 mole of a given substance by 1 K is called molar heat capacity of a substance and is calculated by the formula
c μ = Q ν Δ T ,
where Q is the amount of heat required to heat a certain amount of a substance; ν is the amount of substance; ΔT is the change in temperature of the specified amount of substance when heated.
In the International System of Units, the molar heat capacity of a substance is measured in joules per mol-kelvin (1 J/(mol ⋅ K)).
The amount of heat required to heat some amount of substance, is determined by the product
Q = c µ νΔT .
Molar and specific heat capacities of a substance interconnected expression
c µ = Ms beats,
where c µ is the molar heat capacity of the substance; M is the molar mass of the substance; c sp - specific heat capacity of the substance.
Example 14 Iron and lead balls have the same diameter. How many times greater is the heat capacity of an iron ball than that of a lead ball? The specific heat capacities of iron and lead are 0.46 and 0.13 kJ / (kg ⋅ K), and the densities are 7.80 and 11.5 g / cm 3, respectively.
Solution . The heat capacities of the balls are determined by the following formulas:
- iron ball -
C 1 \u003d m 1 c beat1,
where m 1 is the mass of the iron ball; c ud1 - specific heat capacity of iron;
- lead ball -
C 2 \u003d m 2 c beat2,
where m 2 is the mass of the lead ball; c sp2 - specific heat capacity of lead.
The desired ratio is the heat capacities:
C 1 C 2 \u003d m 1 c beat 1 m 2 c beat 2,
which is determined by the ratio of the masses of the iron and lead balls and the ratio of the specific heats of iron and lead.
The masses of the balls are determined by their size and density:
- iron ball -
m 1 \u003d ρ 1 V 1,
where ρ 1 is the density of iron; V 1 - the volume of the iron ball;
- lead ball -
m 2 \u003d ρ 2 V 2,
where ρ 2 - the density of lead; V 2 - the volume of the lead ball.
The balls have the same diameter, so their volumes are the same:
V 1 \u003d V 2 \u003d V \u003d π d 2 6,
where d are the diameters of the iron and lead balls.
Taking into account the latter circumstance, the mass ratio is equal to:
m 1 m 2 = ρ 1 V 1 ρ 2 V 2 = ρ 1 ρ 2 .
Let's substitute m 1 /m 2 into the formula for the ratio of the heat capacities of the iron and lead balls:
C 1 C 2 \u003d ρ 1 c sp 1 ρ 2 c sp 2.
Let's do the calculation:
C 1 C 2 = 7.80 ⋅ 10 3 ⋅ 0.46 ⋅ 10 3 11.5 ⋅ 10 3 ⋅ 0.13 ⋅ 10 3 = 2.4.
The heat capacity of an iron ball is 2.4 times that of a lead ball.
Example 15. When preparing a mixture, a certain mass of sand and four times the mass of cement were poured into the bunker. The specific heat capacities of cement and sand are 810 and 960 J/(kg ⋅ K), respectively. Determine the specific heat capacity of the mixture.
Solution . The specific heat capacity of the mixture is determined by the formula
c beats = Q m Δ T ,
where Q is the amount of heat required to raise the temperature of the mixture by ΔT; m is the mass of the mixture.
The amount of heat required to heat the mixture, -
Q \u003d Q 1 + Q 2,
where Q 1 - the amount of heat required to heat the sand, which is part of the mixture, by ΔT; Q 2 - the amount of heat required to heat the cement, which is part of the mixture, by ΔT.
The amount of heat required for heating:
- sand -
Q 1 \u003d c ud1 m 1 ∆T,
where c ud1 - specific heat capacity of sand; m 1 - mass of sand;
- cement -
Q 2 \u003d c ud2 m 2 ∆T,
where c ud2 - specific heat capacity of cement; m 2 is the mass of cement.
The amount of heat required to heat a mixture of sand and cement is determined by the expression
Q \u003d c beat 1 m 1 Δ T + c beat 2 m 2 Δ T \u003d (c beat 1 m 1 + c beat 2 m 2) Δ T.
The mass of the mixture is the sum of the masses of sand and cement:
m \u003d m 1 + m 2.
Let us substitute the obtained expressions for the amount of heat and mass of the mixture into the formula for the specific heat capacity of the mixture:
c beats \u003d (c beats 1 m 1 + c beats 2 m 2) Δ T (m 1 + m 2) Δ T \u003d c beats 1 m 1 + c beats 2 m 2 m 1 + m 2.
We will transform the resulting expression, taking into account the mass ratio:
m 2 = 4m 1 , i.e. c beats \u003d c beats 1 m 1 + 4 c beats 2 m 1 m 1 + 4 m 1 \u003d c beats 1 + 4 c beats 2 5.
The calculation gives the value:
c beats = 960 + 4 ⋅ 810 5 = 840 J/(kg ⋅ K).
Therefore, the specific heat capacity of the mixture is 840 J/(kg ⋅ K).
