gas mixtures. Heat capacity of gases. Fuel, gas mixtures and heat capacity
1.3. Ideal gas equation of state
The form of the equations of state (1.1) for real substances is rather complicated.
In this regard, simplified physical models of working bodies are used in thermodynamics.
For working fluids in the gaseous state, the simplest and historically the first model is ideal gas. An ideal gas is a gas in which the forces of intermolecular interaction (attraction and repulsion) are absent, and the molecules themselves are considered as material points. An ideal gas obeys the Claiperon equation– Mendeleev
Where m is the mass of gas in the system; μ is the molar mass of the gas; Rμ = 8314 J/(kmol K) is the universal gas constant, which does not depend on the type of gas or on the conditions of its existence. Equation of state (1.2) is obtained by combining Boyle-Mariotte laws And Gay Lussac taking into account Avogadro's law.
Molar mass μ = m/n, kg/mol, is the mass of a substance taken in the amount of one mole. 1 mol is a unit of the amount of a substance that contains as many particles as 12 grams of carbon. Amount of substance– n = N/N A, mole, where N is the number of particles (atoms, molecules), N A ≈ 6.02 10 23 - the number of atoms in 12 grams of carbon ( Avogadro's number). Numerically μ = Мr 10 -3 , where Mr is the relative molecular weight of the substance ( tab. Mendeleev) (for example: =2 kg/kmol).
In specific terms (i.e. for 1 kg of gas), equation (1.2) will have the form
Where R– specific gas constant, J/(kg K).
Let us explain the physical meaning of the specific gas constant R. For the first state equation (1.2) will be written as , for the second state at the same pressure - . Subtract the first equation from the second equation and find that , i.e. The specific gas constant is the work done by 1 kg of gas when it is heated by 1 degree at constant pressure.
Experimental data show that a real gas is the closer to an ideal one, the lower its density. In other words, with a decrease in the volume of a given amount of gas, caused by an increase in pressure or a decrease in temperature, any gas will give more and more deviations from the properties of an ideal gas. Thus, under the concept of "ideal gas" fit all real gases at high temperatures and low pressures.
1.4. Mixes ideal gases
In practice, the working fluid is often a mixture of homogeneous gases (for example, air), each of which can be considered ideal and which do not enter into chemical reactions.
Such a mixture is also an ideal gas and obeys the equation of state (1.2) for the mixture
Where R=R μ / μ is the gas constant of the mixture; μ is the average (apparent) molar mass of the mixture(a conditional value referring to a homogeneous representative gas, in which the number of molecules and the total mass are equal to the number of molecules and the mass of the mixture).
The mass of the mixture is equal to the mass of all constituent components
The main law that determines the behavior of a gas mixture is dalton's law:
each individual gas behaves in a gas mixture as if it alone, at the temperature of the mixture, occupies the entire volume of the mixture,
each individual gas entering the gas mixture has the same pressure as it would have if it alone occupied the entire volume of the gas mixture.
Hence the value R(absolute mixture pressure) is defined as
Where R i – partial pressure i th component, i.e. pressure that would i th component, if it alone occupied the entire volume of the mixture at the same temperature ().
The composition of the mixture is given by mass or mole fractions of the constituent components of the mixture, as well as by volume fractions (concentrations).
Mass fraction is the ratio of the mass of each gas to the total mass of the mixture: .
Equation (1.3) implies that .
Volume fraction is called the ratio partial volume(the volume that the gas would occupy if its pressure and temperature were equal to the pressure and temperature of the gas mixture, i.e.) to the total volume of the gas mixture: . From the definitions of partial pressure and volume and the constancy of temperature, it follows:
![]() (1.5)
(1.5)
Summing up the last equality over all components of the mixture, we obtain , i.e. the sum of the partial volumes of the gases that make up the mixture is equal to the volume of the mixture of gases.
The sum of volume fractions is equal to one: .
mole fraction component is called the ratio of the amount of substance of each gas n i to the amount of substance of the mixture of gases n. From the relations and and Avogadro's law(in equal volumes of different ideal gases at the same temperatures and the same pressures, an equal number of molecules are enclosed, i.e.) follows:
those. specifying a mixture by mole fractions is equivalent to specifying its volume fractions.
Mass and volume fractions are related by the ratio:
![]() .
.
If the mixture is given by mass fractions g i, then the gas constant of the mixture and the molar mass of the mixture are calculated as follows:
![]()
![]()
If the mixture is given by volume fractions r i, then the formulas for calculating the molar mass of the mixture and the gas constant of the mixture are as follows:
![]() (1.6)
(1.6)
Partial pressures and volumes of gases are determined by boyle's law–marriotte(at a constant gas temperature, the product of the gas pressure and its volume is a constant value, i.e.):
![]() .
.
Control questions
International System of Units (SI) and its base units.
Basic thermodynamic parameters and their dimensions.
What is meant by the equilibrium state of a thermodynamic system?
The concept of equilibrium and non-equilibrium states.
What is the equation of state of a system?
Geometric interpretation of the state of the system, thermodynamic process.
What is an ideal gas? What is the difference between an ideal gas and a real one?
What is the gas constant? Its physical meaning, dimension and methods of definition.
2. Heat capacity
The message of heat to the working body in any process causes a change in its state and, in the general case, is accompanied by a change in temperature. The change in temperature, as well as any other parameter of the state of the working fluid, does not depend on the type of process, but depends on its initial ( 1 ) and final ( 2 ) states, i.e. (for an elementary process, the change in a parameter is replaced by its differential).
The ratio of the amount of heat δ Q communicated to the body to a change in body temperature dT in an elementary thermodynamic process is called true heat capacity bodies in this process:
The subscript here indicates the fact that the heat capacity, like heat, depends on the nature of the process. In addition, the heat capacity depends on the amount of the body and its thermodynamic state. It should also be noted that in this process the chemical composition of the body does not change, there is no transition of a substance from one state of aggregation to another, there is no dissolution of components, etc.
Depending on the chosen unit of quantity of a substance, mass, volume and molar specific heat capacities are distinguished:
The concept of mass heat capacity is most often used in practice.
2.1. True and average heat capacity
The heat capacity of a real substance is not a constant value. It changes with temperature, and this dependence can be very significant (Fig. 2.1).
The specific amount of process heat is calculated by the formula
The following methods are used to determine this value:
according to the results of the experiment, the dependence of heat capacity on temperature is represented as an approximation polynomial
where are the approximation coefficients. These coefficients are given in the reference literature. Then
in practical calculations in the temperature range t 1 , t 2 heat capacity is considered a constant value equal to
called average heat capacity in this interval, in contrast to the true heat capacity introduced in (2.1). Reference data give average heat capacities from 0 to fixed temperature t obtained empirically, i.e.
Average heat capacity in the temperature range t 1 , t 2 according to these reference data can be calculated by the formula
Then specific quantity process heat is defined as
2.2. Isochoric and isobaric heat capacities
are of great importance in thermodynamics.
isochoricheat capacity
equal to the ratio of the amount of heat in the process at a constant volume to the change in body temperature,
isobaricheat capacity
equal to the ratio of the amount of heat in the process at constant pressure to the change in body temperature.
In thermotechnical calculations, tables are used that show numerical values obtained experimentally for specific isobaric and isochoric heat capacities for various substances depending on temperature.
2.3. Heat capacity of gas mixtures
In calculations, it is often necessary to deal with mixtures of gases, and the heat capacities are given in the tables only for individual gases.
If the mixture of gases is given by mass fractions g i, then the specific mass heat capacities of the mixture are determined by the formulas
![]()
If the mixture of gases is given by volume fractions r i, then the specific volumetric heat capacities of the mixture are determined by the formulas
![]()
Control questions
Write down the units of specific heat capacity.
How is the concept of average heat capacity introduced?
3. The law of conservation and transformation of energy
The concept of energy is associated with the movement of matter.
Energy can take many forms - mechanical work, heat, chemical energy, energy of electric and magnetic fields.
In a thermodynamic process, the interaction of the environment and a closed thermodynamic system is carried out by exchanging energy in the form of heat and mechanical work.
3.1. Internal energy
Any environment has some margin internal energyU(J), which in technical thermodynamics is represented as the sum of the kinetic and potential energies of the molecules and atoms of the medium. The kinetic energy of these particles is determined by their speed and mass, while the potential energy is determined by the forces of interaction between them, which depend on their relative position. The internal energy of a system is the energy contained in the system itself. This means that the internal energy can characterize the state of the body along with the quantities R, V, T.
The internal energy has the additivity property, i.e. the internal energy of a complex system is equal to the sum of the internal energies of its constituent parts:
The internal energy of 1 kg of a substance is called specific internal energy u= U/ m (J/kg).
For most technical applications of thermodynamics, it is not the absolute value that matters U, and the change in this value. Therefore, the quantitative definition of the internal energy of a homogeneous system is often determined with respect to some conventionally chosen standard state.
3.2. Law of conservation of energy in thermodynamics
Performing a thermodynamic process, a closed system interacts with the external environment (external bodies and fields), i.e. exchanges energy. In technical thermodynamics, two types of energy transfer are considered - by transferring heat and by performing mechanical work.
Heat transfer occurs between bodies of different temperatures and brought into contact, or between bodies with different temperatures, located at a distance, by means of electromagnetic waves ( thermal radiation). Broadcast warmth occurs at the molecular (microphysical) level without visible movement of bodies.
The transfer of energy in the form of work occurs with the movement of the entire body or part of it in space. With this method, the body either moves in a force field, or changes its volume under the influence of external pressure. Job is a macrophysical form of energy transfer.
It should be noted that heat and work, unlike the energy of a body, are not functions of its state, but depend on the type of process, determine this process, i.e. are functions of the process itself.
Numerous experiments and observations led to the discovery of a fundamental law of nature - the law of conservation of energy: energy in nature does not arise from nothing and does not disappear, or the amount of energy is unchanged, it only changes from one form to another, or
Where Q is the heat involved in the process; L- the work being done; Δ E is the change in the energy of the system. Here and below, we agree to consider:
work positive if this work is done by the working body, and negative, if work is done on the working body of the system;
heat supplied to the body positive, and the allotted - negative
in addition, a change in any state parameter in the final process will be denoted by the symbol Δ (delta), and in the elementary process - d(differential of the corresponding parameter). For any finite thermodynamic process, the change in the state parameter (pressure, temperature, internal energy, enthalpy, entropy, etc.) does not depend on the type of process, but is determined by the initial and final states.
In general, a thermodynamic system has an internal energy U, kinetic energy (in thermodynamics, the system is considered as a whole moving at a speed w) – mw 2 /2, potential - mgz (z is the height at which the system is located). Change in total energy in the process 1 –2 can be imagined as:
The work done in this process is the sum of the work L about, associated with a change in the volume of the working fluid of the system (deformation work), work L dv on the movement of the system in space, technical work L those (when moving the system in various technical devices, for example, in an engine, steam boiler, compressor, etc.), work against friction forces L tr:
L \u003d L about + L dv + L those + L tr.
The heat involved in the process is the sum of the heat Q external, involved in the exchange with the external environment, and the heat of friction Q tr:
Q = Q external + Q tr.
Given that Q tr = L tr (numerous experiments have shown that the work of the flow expended on overcoming friction is completely converted into heat perceived by the flow), the law of conservation of energy in thermodynamics for 1 kg of a working homogeneous substance (i.e., in specific mass values) in an elementary process can be written :
![]() , (3.1)
, (3.1)
(external index omitted as unnecessary).
It should be emphasized that on the right side of (3.1) under the sign of the differential are state functions, and the rest are quantities that depend on the nature of the thermodynamic process. Since work and heat are process functions, and not state functions, the sign δ denotes only the fact that in an elementary process the quantities following it are arbitrary infinitesimal, and not increments of any specific functions. Thus, δ q and δ l-elementary (i.e., corresponding to infinitesimal changes in the state of the system) amounts of heat and work.
If the thermodynamic system does not change its position in space and the only type of work is the work associated with a change in the volume of the system, then equation (3.1) takes a simpler form:
Job δ l about is performed either against the forces of external pressure and, then, the volume of the working fluid expands, or, conversely, the external environment performs work on the body, compressing it. For equilibrium processes, when the pressure of the medium is equal to the pressure in the working fluid, this work is calculated as Rdv, Where v is the specific volume of the working substance.
The equation
called the first law of thermodynamics: the heat supplied to a closed thermodynamic system at rest is spent on changing the internal energy of the system and on doing work to change the volume of the system. The balance relation (3.2) is also called lectures By theoretical literature. 1. Baroque: ...
L. A. Eliseeva © Federal State Budgetary Institution of Science State Public Scientific and Technical Library of the Siberian Branch of the Russian Academy of Sciences, 2013
PointerRandom walks / A.N. Borodin, I. A. Ibragimov; under... 241. Dorogokupets P.I. Thermodynamics minerals and mineral equilibria ... catalogues: compendium lectures By course "Reference ... state technical university. Series, Technical Sciences. - ...
N. V. Basova [and others]; ed. N. V. Basova. Rostov n/a: Phoenix, 2008
TextbookCourse) Written D. T. Synopsis lectures By higher mathematics [Text]: ... Technical thermodynamics Rudobashta, S.P. Heat engineering [Text]: textbook for students. universities studying By... 2008. - 204 p. 10 Borodin, I. F. Automation of technological processes...
List of scientific works of the Treasury for the period 2008 - June 1, 2013 (1)
Document... "X-ray methods of research" Lectures By clinical oncology. Almaty... of the second law thermodynamics» 2-International... and expert review technical equipping healthcare organizations ... Academician of the Russian Academy of Medical Sciences Yu.I. Borodin, Bishkek, 2009, p. ...
In engineering practice, one often has to deal not with homogeneous gases, but with mixtures of chemically unrelated gases. Examples of gas mixtures are: atmospheric air, natural gas, gaseous products of combustion of fuels, etc.
For gas mixtures, the following provisions are valid.
1. Each gas entering the mixture has a temperature, equal to temperature mixtures.
2. Any of the gases included in the mixture is distributed throughout the volume of the mixture and therefore the volume of each gas is equal to the volume of the entire mixture.
3. Each of the gases included in the mixture obeys its own equation of state.
4. The mixture as a whole is like a new gas and obeys its own equation of state.
The study of gas mixtures is based on Dalton's law, according to which, at a constant temperature, the pressure of the mixture is equal to the sum of the partial pressures of the gases included in the mixture:
where p cm is the pressure of the mixture;
p i - partial pressure of the i-th gas included in the mixture;
n is the number of gases included in the mixture.
The partial pressure is the pressure that the gas entering the mixture will exert if it alone occupies the entire volume of the mixture at the same temperature.
Methods for setting gas mixtures
The composition of the gas mixture can be specified by mass, volume and mole fractions.
Mass fractions. The mass fraction of any gas included in the mixture is the ratio of the mass of this gas to the mass of the mixture.
m 1 \u003d M 1 / M cm; m 2 \u003d M 2 / M cm; ..........; m n \u003d M n / M cm,
where m 1 , m 2 , ..., m n - mass fractions of gases;
M 1 , M 2 , ..., M n - masses of individual gases;
M cm is the mass of the mixture.
It is easy to see that 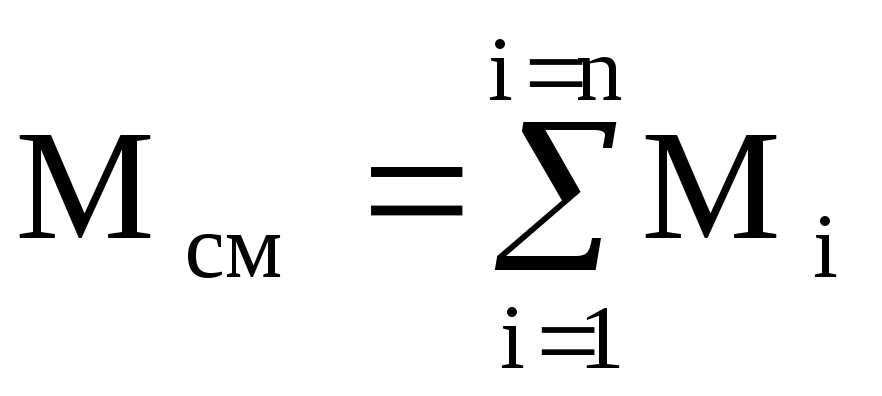 And
And 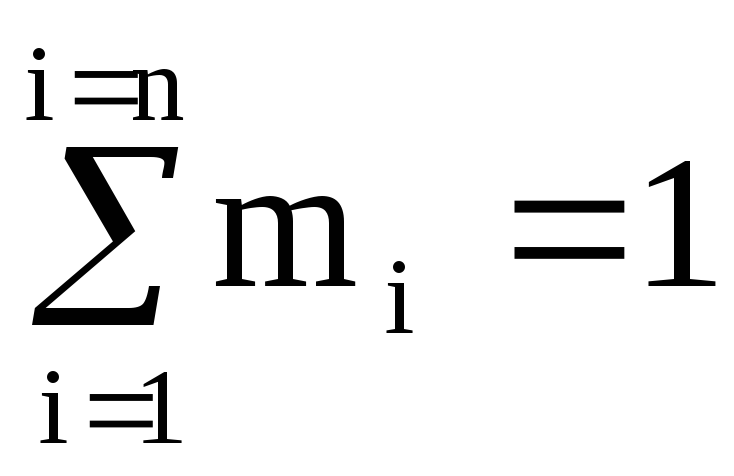 (100%).
(100%).
Volume shares. The volume fraction of any gas included in the mixture is the ratio of the reduced (partial) volume of this gas to the volume of the mixture.
r 1 \u003d V 1 / V cm; r 2 \u003d V 2 / V cm; ........., r n = V n / V cm;
where V 1 , V 2 , ..., V n - reduced volumes of gases;
V cm is the volume of the mixture;
r 1 , r 2 , ..., r n - volume fractions of gases.
The reduced volume is the volume of gas under the conditions of the mixture (at the temperature and pressure of the mixture).
The reduced volume can be represented as follows: if all gases except one are removed from the vessel containing the mixture, and the remaining gas is compressed to the pressure of the mixture while maintaining the temperature, then its volume will be reduced or partial.
It can be proved that the volume of the mixture will be equal to the sum of the reduced volumes of gases.
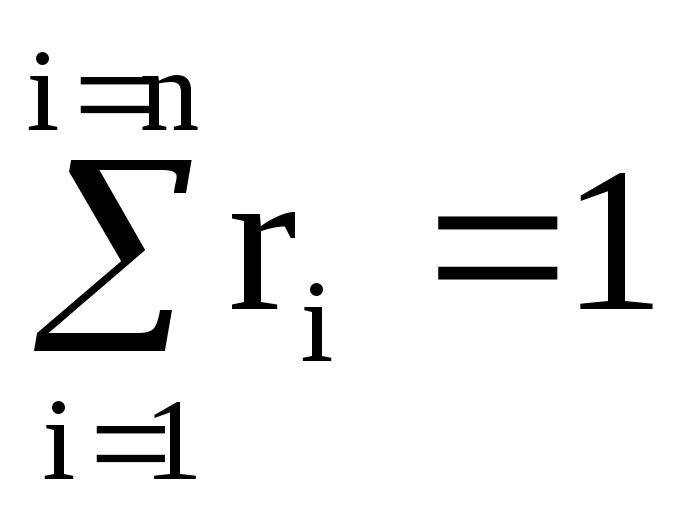 (100%).
(100%).
Mole fractions. The mole fraction of any gas included in a mixture is the ratio of the number of kilomoles of this gas to the number of kilomoles of the mixture.
r 1 \u003d n 1 / n cm; r 2 \u003d n 2 / n cm; ........., r n \u003d n n / n cm,
where r 1 , r 2 , ..., r n - mole fractions of gases;
n cm is the number of kilomoles of the mixture;
n 1 , n 2 , ..., n n is the number of kilomoles of gases.
Specifying a mixture by mole fractions is identical to specifying a mixture by volume fractions, i.e. molar and volume fractions have the same numerical values for each gas included in the mixture.
Gas constant and apparent (average) molecular weight of the mixture. To calculate the gas mixture constant given by mass fractions, we write the equations of state:
for mixture
p cm × V cm = M cm R cm T; (1.9)
for gases
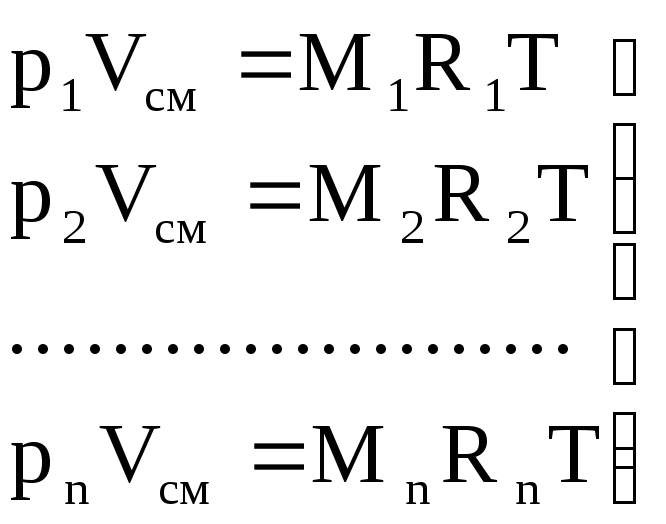 .
(1.10)
.
(1.10)
We sum the left and right parts of equations (1.10)
(p 1 + p 2 + .... + p n) V cm = (M 1 R 1 + M 2 R 2 + ..... + M n R n) T.
Because 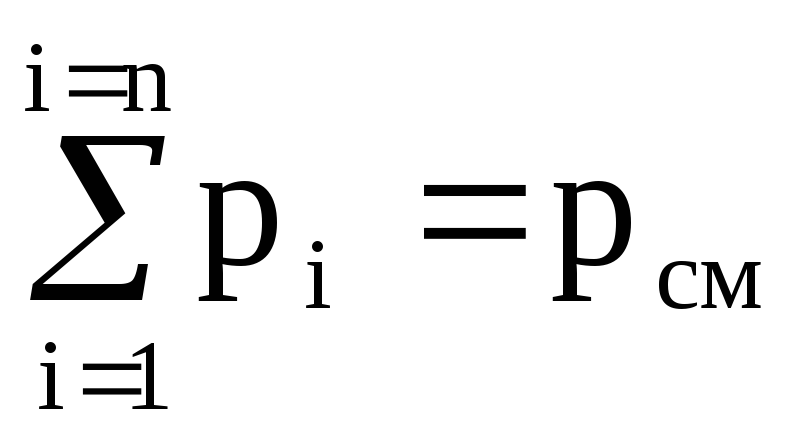 ,
,
then p cm V cm = (M 1 R 1 + M 2 R 2 + ..... + M n R n) T. (1.11)
Equations (1.9) and (1.11) imply that
M cm R cm T \u003d (M 1 R 1 + M 2 R 2 + ..... + M n R n) T.
R cm \u003d M 1 / M cm R 1 + M 2 / M cm R 2 + ...... + M n / M cm R n \u003d
M 1 R 1 + m 2 R 2 + ...... + m n R n
or 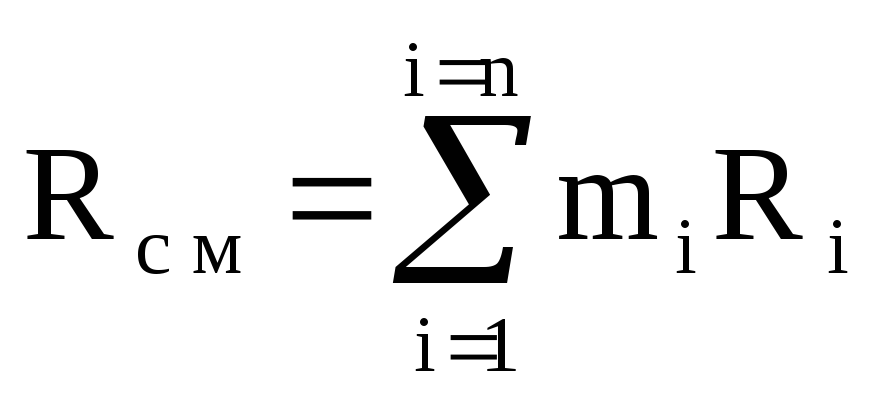 ,
(1.12)
,
(1.12)
where R cm is the gas constant of the mixture.
Since the gas constant of the i-th gas
R i = 8314 / m i ,
then equation (1.12) is rewritten as follows:
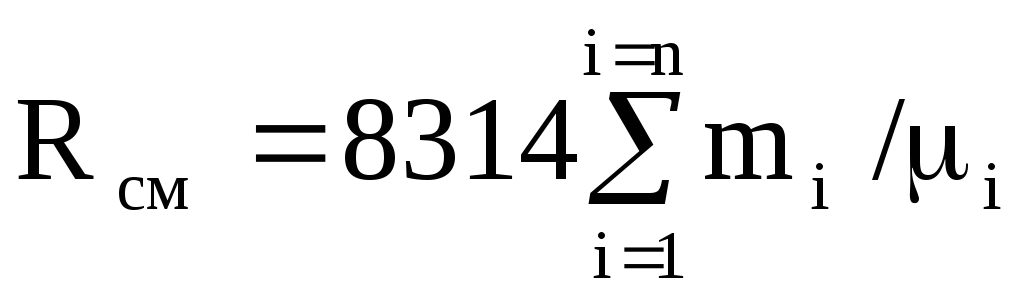 .
(1.13)
.
(1.13)
When determining the parameters of a gas mixture, it is convenient to use a certain conditional value called the apparent (average) molecular weight of the gas mixture. The concept of the apparent molecular weight of a mixture allows us to conventionally consider the mixture as a homogeneous gas, which greatly simplifies the calculations.
For a separate gas, the expression
By analogy, for a mixture, we can write
m cm R cm = 8314, (1.14)
where m cm is the apparent molecular weight of the mixture.
From equation (1.14), using expressions (1.12) and (1.13), we obtain
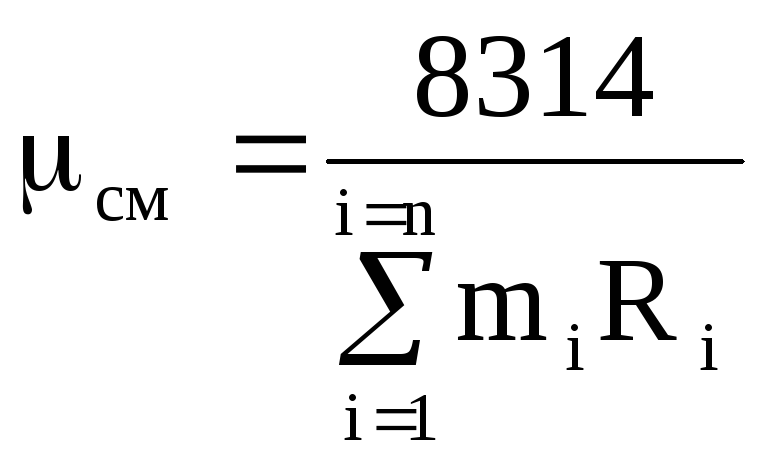 ,
(1.15)
,
(1.15)
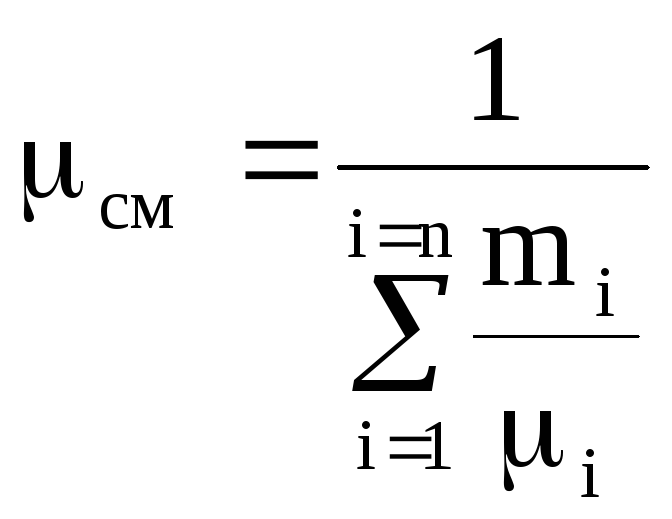 .
(1.16)
.
(1.16)
Arguing in this way, one can obtain formulas for calculating R cm and m cm through volume fractions, formulas for converting mass fractions into volume fractions and, conversely, volume fractions into mass fractions, formulas for calculating the specific volume of a mixture u cm and mixture density r cm through mass and volume fractions and, finally, formulas for calculating the partial pressures of gases included in the mixture, through volume and mass fractions. We present these formulas without derivation in the table.
Formulas for calculating gas mixtures
|
Setting the composition of the mixture |
Transfer from one composition to another |
Density and specific volume of the mixture |
Apparent molecular weight of the mixture |
Gas mixture constant |
Partial pressure |
|
Mass fractions |
|
|
|
|
|
|
Volume fractions |
|
|
|
|
|
Heat capacity of gases
The heat capacity of a body is the amount of heat required to heat or cool the body by 1 K. The heat capacity of a unit amount of a substance is called the specific heat capacity.
So, the specific heat capacity of a substance is the amount of heat that must be imparted or subtracted from a unit of a substance in order to change its temperature by 1 K in this process.
Since only specific heat capacities will be considered in what follows, we will refer to the specific heat capacity simply as the heat capacity.
The amount of gas can be given by mass, volume and number of kilomoles. It should be noted that when setting a gas volume, this volume is brought to normal conditions and measured in normal cubic meters (nm 3).
Depending on the method of setting the amount of gas, the following heat capacities are distinguished:
c - mass heat capacity, J / (kg × K);
c¢ - volumetric heat capacity, J / (nm 3 × K);
c m - molar heat capacity, J / (kmol × K).
Between these heat capacities there are the following relationships:
c = c m / m; with m = with × m;
с¢ = с m / 22.4; with m = s¢ × 22.4,
from here 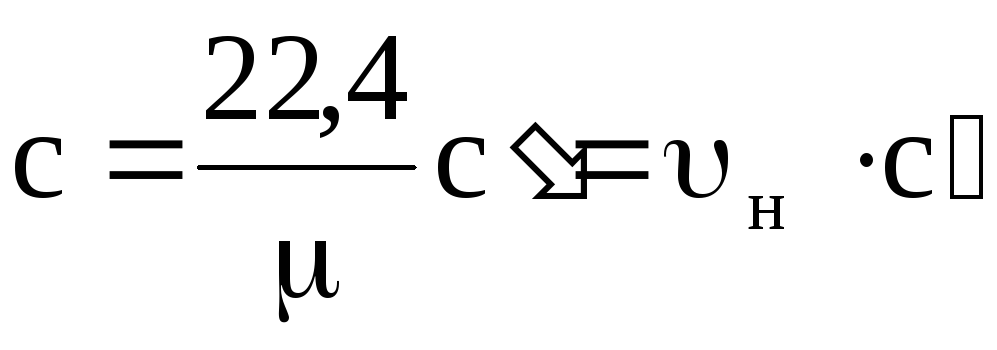 ; s¢ = s × r n,
; s¢ = s × r n,
where u n and r n - specific volume and density under normal conditions.
Isochoric and isobaric heat capacities
The amount of heat imparted to the working fluid depends on the features of the thermodynamic process. Two types of heat capacity are of practical importance depending on the thermodynamic process: isochoric and isobaric.
The heat capacity at u = const is isochoric.
c u - mass isochoric heat capacity,
c¢ u is the volumetric isochoric heat capacity,
c m u is the molar isochoric heat capacity.
The heat capacity at p = const is isobaric.
c p - mass isobaric heat capacity,
c¢ р - volumetric isobaric heat capacity,
c m p - molar isobaric heat capacity.
With the same change in temperature in the process carried out at p = const, more heat is consumed than in the process at u = const. This is explained by the fact that at u = const the heat imparted to the body is spent only on changing its internal energy, while at p = const the heat is spent both on increasing the internal energy and on performing the work of expansion. The difference between the mass isobaric and mass isochoric heat capacities according to the Mayer equation
c p - c u=R. (1.17)
If the left and right sides of equation (1.17) are multiplied by the kilomole mass m, then we get
c m p - c m u= 8314 J/(kmol×K) (1.18)
In thermodynamics and its applications, the ratio of isobaric and isochoric heat capacities is of great importance:
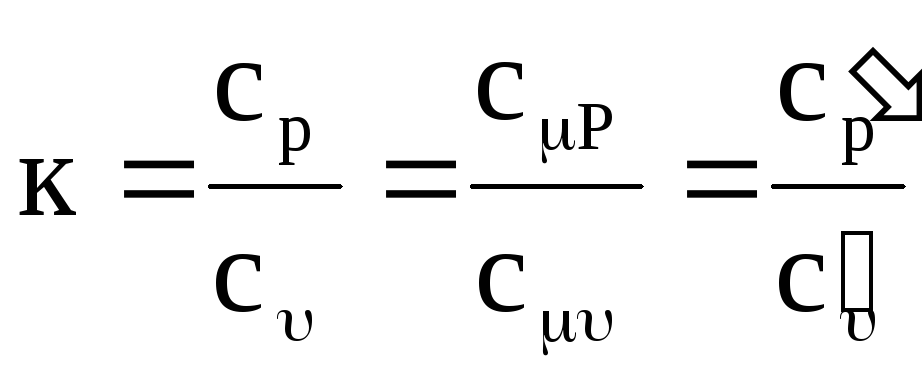 ,
(1.19)
,
(1.19)
where k is the adiabatic exponent.
Calculations show that for monatomic gases k » 1.67, diatomic gases k » 1.4, and triatomic gases k » 1.29.
It is easy to see that the value To temperature dependent. Indeed, it follows from equations (1.17) and (1.19) that
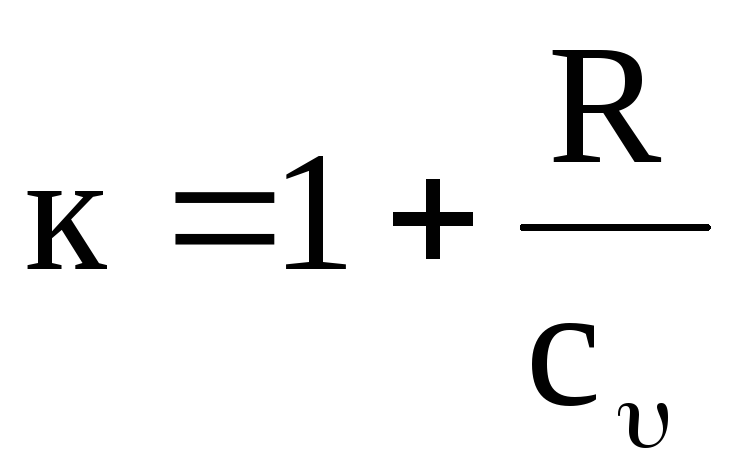 ,
(1.20)
,
(1.20)
and from equations (1.18) and (1.19)
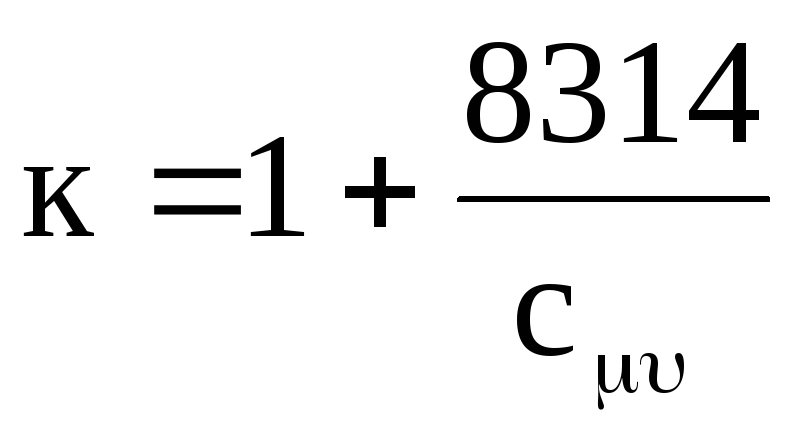 .
(1.21)
.
(1.21)
Since the heat capacities increase with increasing gas temperature, the value of k decreases, approaching unity, but always remains greater than it.
Knowing the value of k, one can determine the value of the corresponding heat capacity. So, for example, from equation (1.20) we have
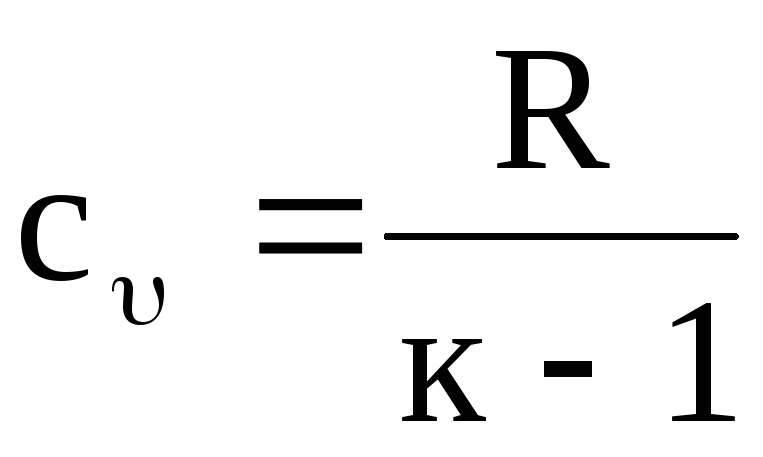 ,
(1.22)
,
(1.22)
and since with p = k × s u, then we get
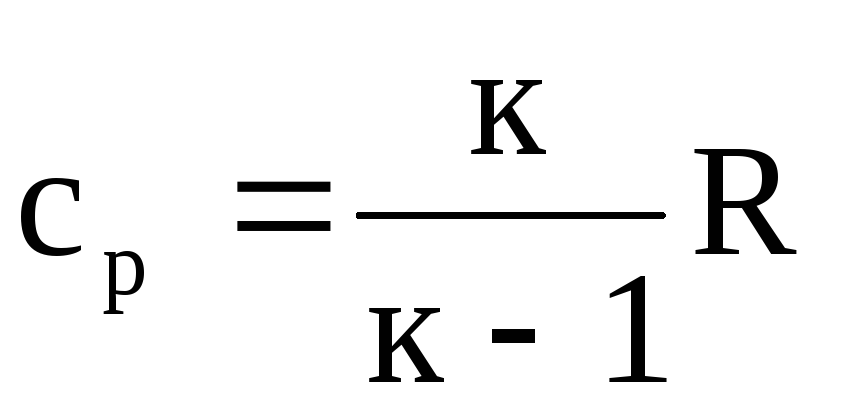 .
(1.23)
.
(1.23)
Similarly, for molar heat capacities, from equation (1.21) we obtain
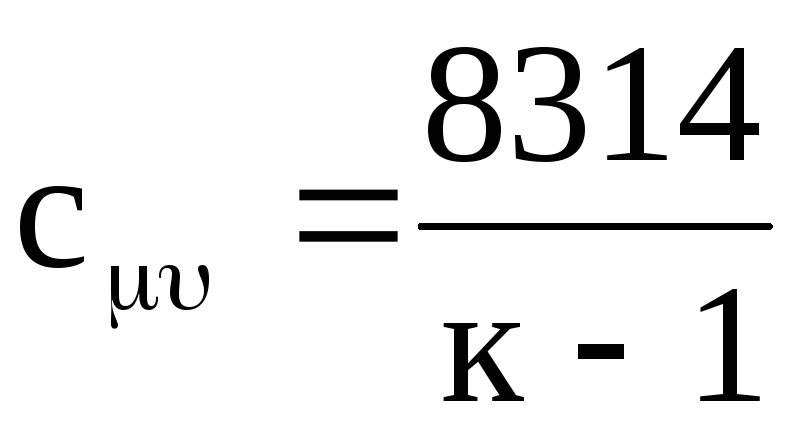 .
(1.24)
.
(1.24)
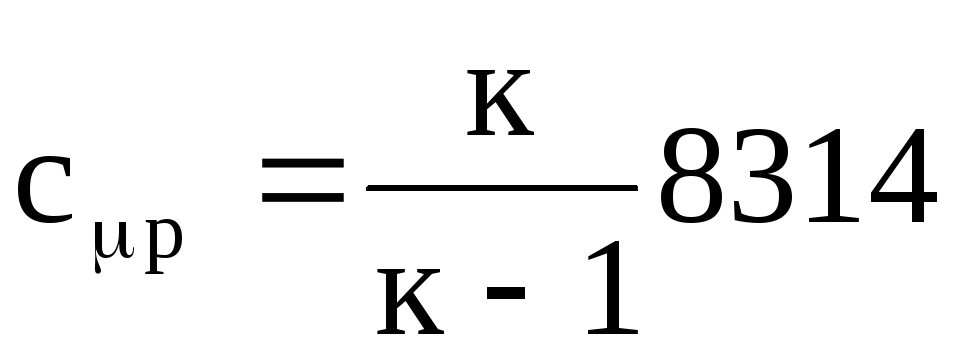 .
(1.25)
.
(1.25)
Average and true heat capacity
The heat capacity of gases depends on temperature and, to some extent, on pressure. The dependence of heat capacity on pressure is small and is neglected in most calculations. The dependence of heat capacity on temperature is significant and must be taken into account. This dependence is quite accurately expressed by the equation
c = a + V t + et 2 , (1.26)
where a, V and e are values that are constant for a given gas.
Often in thermal engineering calculations, the nonlinear dependence (1.26) is replaced by a linear one:
c = a + V t. (1.27)
|
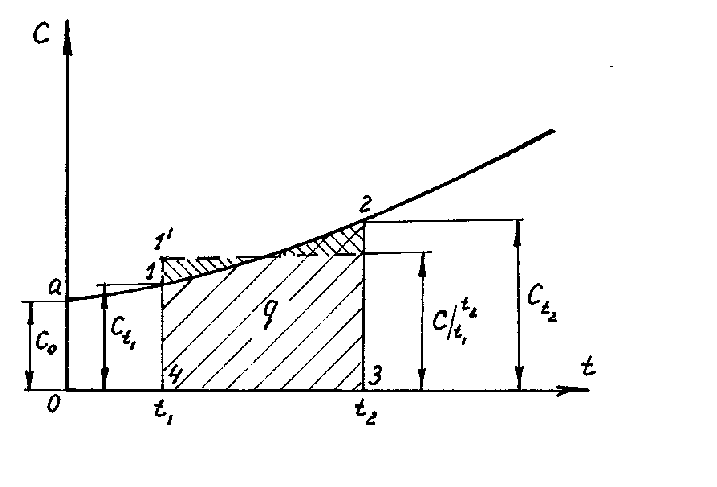
If we construct graphically the dependence of heat capacity on temperature according to equation (1.26), then this will be a curvilinear dependence (Fig. 1.4). As shown in the figure, each temperature value has its own heat capacity value, which is commonly called the true heat capacity. Mathematically, the expression for the true heat capacity is written as follows:
|
|
|
|
Therefore, the true heat capacity is the ratio of an infinitesimal amount of heat dq to an infinitesimal change in temperature dt. In other words, the true heat capacity is the heat capacity of the gas at a given temperature. On fig. 1.4, the true heat capacity at a temperature t 1 is indicated with t1 and is depicted as a segment 1-4, at a temperature t 2 - with t2 and is depicted as a segment 2-3. From equation (1.28) we get dq=cdt. (1.29) In practical calculations, we always determine the amount of heat at the final change |
temperature. It is obvious that the amount of heat q, which is reported to a unit amount of a substance when it is heated from t 1 to t 2, can be found by integrating (1.29) from t 1 to t 2.
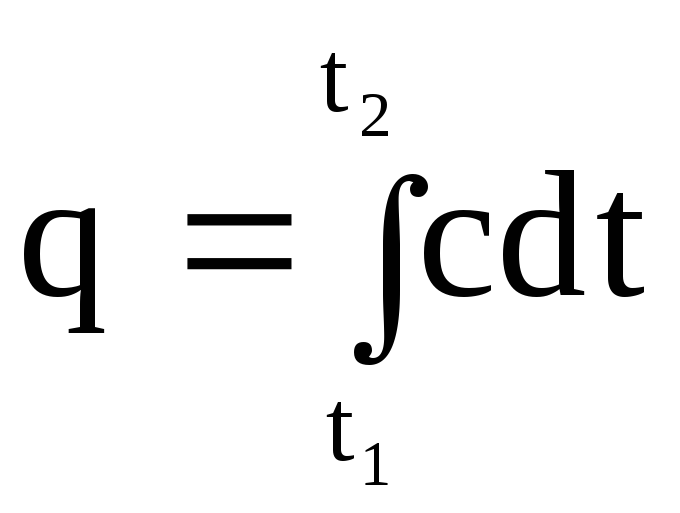 .
(1.30)
.
(1.30)
Graphically, the integral (1.30) is expressed by the area 4-1-2-3. If in expression (1.30) we substitute the value of the true heat capacity according to the linear dependence (1.27), then we obtain
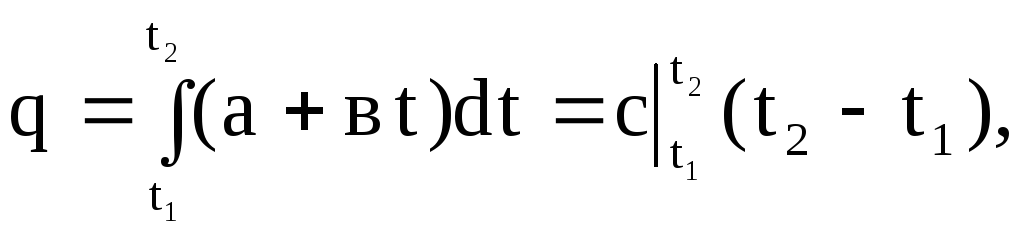 (1.31)
(1.31)
Where 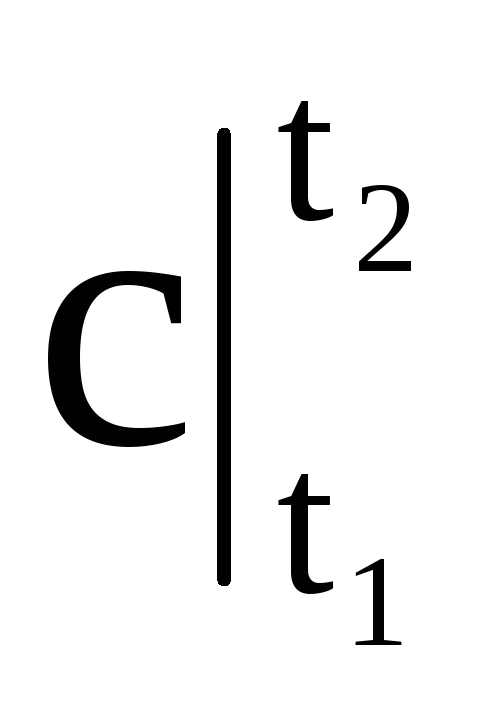 - average heat capacity in the temperature range from t 1 to t 2.
- average heat capacity in the temperature range from t 1 to t 2.
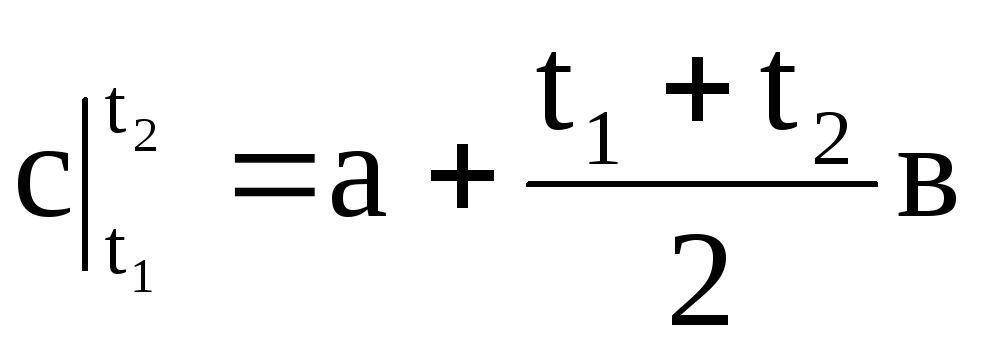 ,
(1.32)
,
(1.32)
Therefore, the average heat capacity is the ratio of the final amount of heat q to the final temperature change t 2 - t 1:
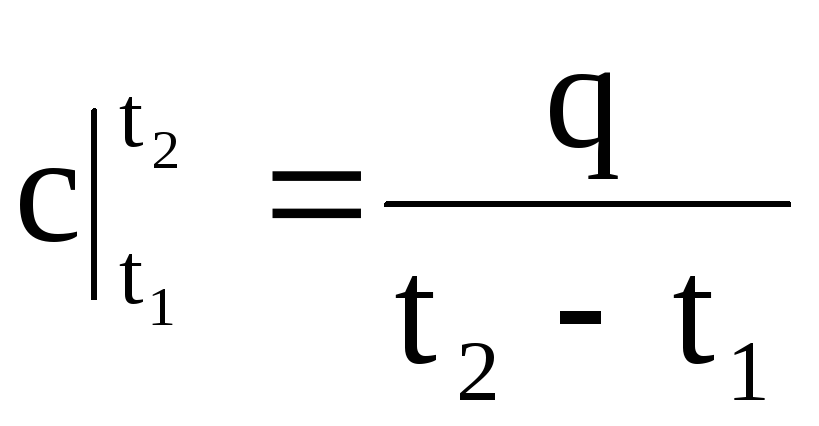 .
(1.33)
.
(1.33)
If, on the basis of 4-3 (Fig. 1.4), a rectangle 4-1¢-2¢-3 is constructed, equal in size to the figure 4-1-2-3, then the height of this rectangle will be equal to the average heat capacity, where 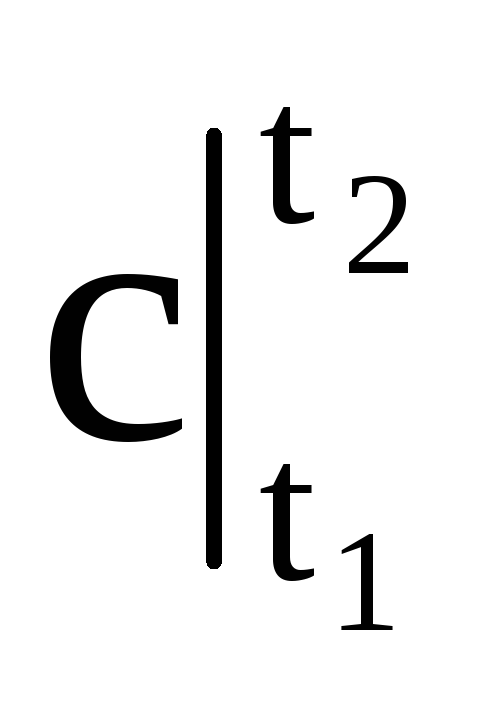 is in the temperature range t 1 - t 2 .
is in the temperature range t 1 - t 2 .
Usually, the values of the average heat capacities are given in tables of thermodynamic properties of substances. However, to reduce the volume of these tables, they provide the values of the average heat capacities determined in the temperature range from 0 ° C to t ° C.
If it is necessary to calculate the value of the average heat capacity in a given temperature range t 1 - t 2, then this can be done as follows.
The area 0a14 under the curve c \u003d f (t) (Fig. 1.4) corresponds to the amount of heat q 1 required to increase the gas temperature from 0 ° C to t 1 ° C.
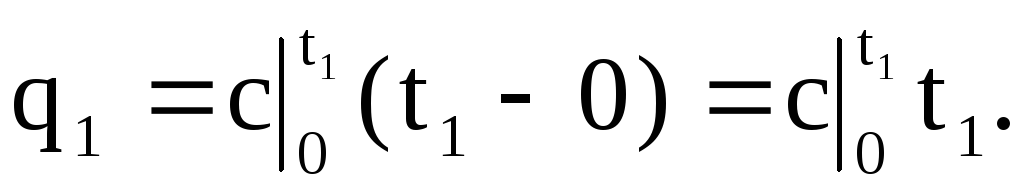
Similarly, the area 0a23 corresponds to q 2 when the temperature rises from 0 o C to t 2 o C:
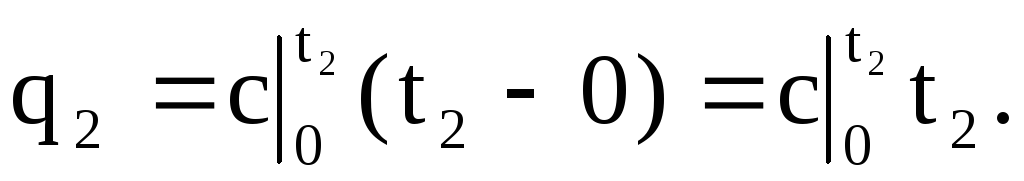
Thus, q \u003d q 2 - q 1 (area 4123) can be represented as
![]() (1.34)
(1.34)
Substituting the value of q according to (1.34) into expression (1.33), we obtain the formula for the average heat capacity in any temperature range:
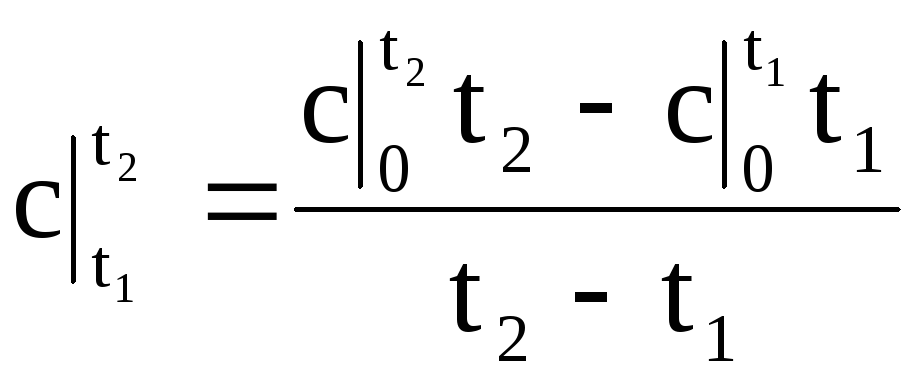 .
(1.35)
.
(1.35)
Thus, the average heat capacity can be calculated from the tabular average heat capacities using equation (1.35). Moreover, we obtain a nonlinear dependence c = f(t). You can also find the average heat capacity using equation (1.32) using a linear relationship. Values a and V in equation (1.32) for various gases are given in the literature.
The amount of heat supplied or removed from the working fluid can be calculated using any of the equations:
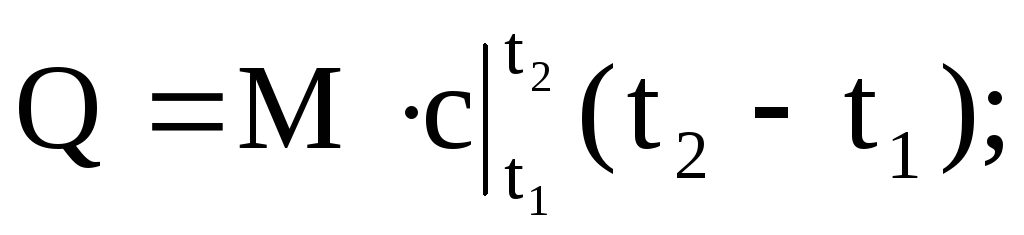 (1.36)
(1.36)
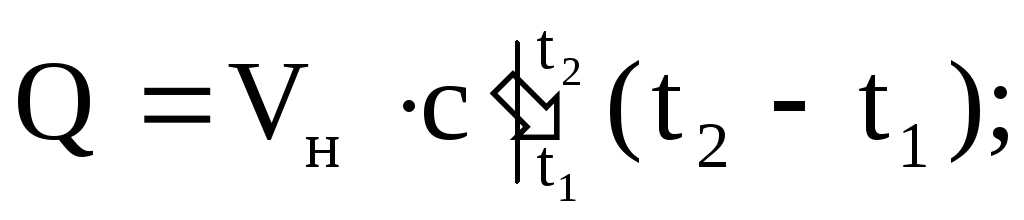 (1.37)
(1.37)
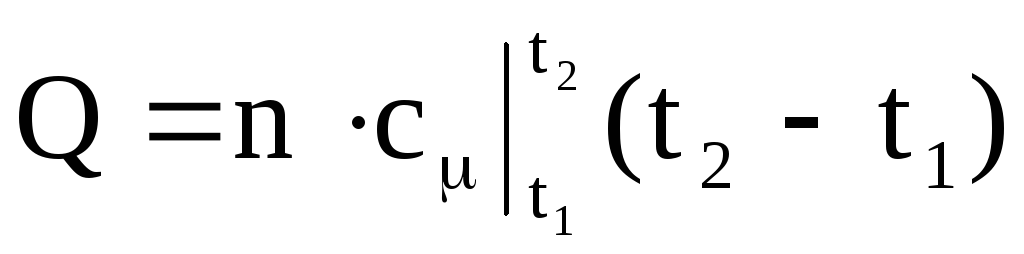 ,
(1.38)
,
(1.38)
Where 
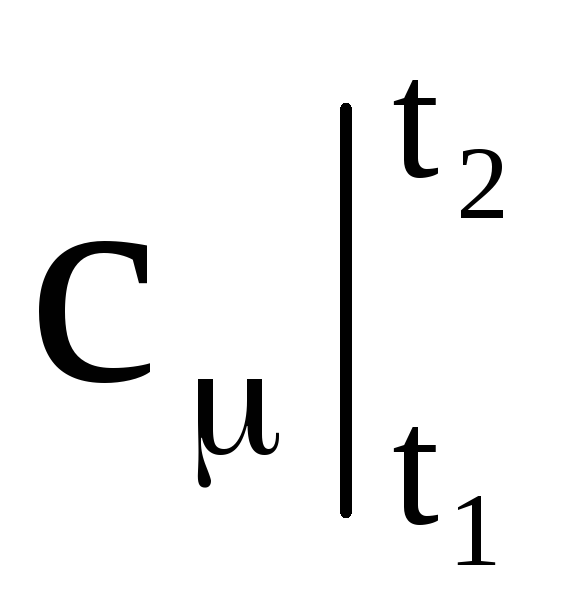 - respectively, the average mass, volume and molar heat capacity; M is the mass of gas; n is the number of kilomoles of gas; V n - the volume of gas under normal conditions.
- respectively, the average mass, volume and molar heat capacity; M is the mass of gas; n is the number of kilomoles of gas; V n - the volume of gas under normal conditions.
The volume of gas V n can be found as follows. Having written the equation of state for the given conditions: pV = MRT and for normal conditions: p n V n = MRT n, we attribute the second equation to the first:
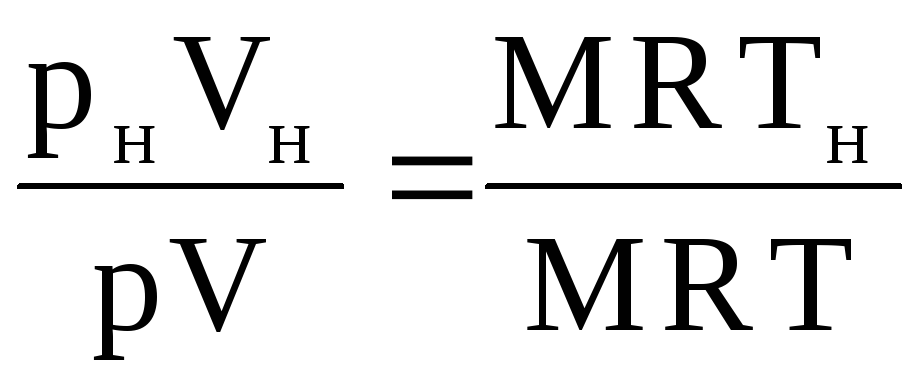 ,
,
from here 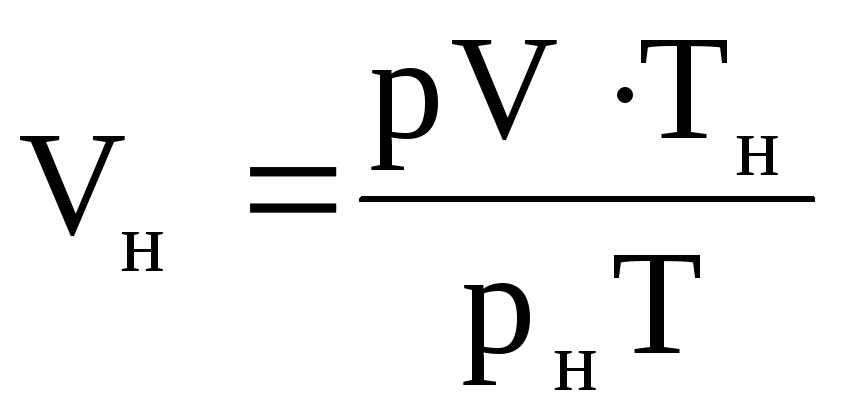 .
(1.39)
.
(1.39)
Heat capacity of gas mixtures
The heat capacity of a gas mixture can be calculated if the composition of the mixture is given and the heat capacities of the components included in the mixture are known.
To heat a mixture of mass M cm by 1 K, the temperature of each of the components must also be increased by 1 K. At the same time, the amount of heat equal to c i M i is spent on heating the i-th component of the mixture with mass М i . For the entire mixture, the amount of heat 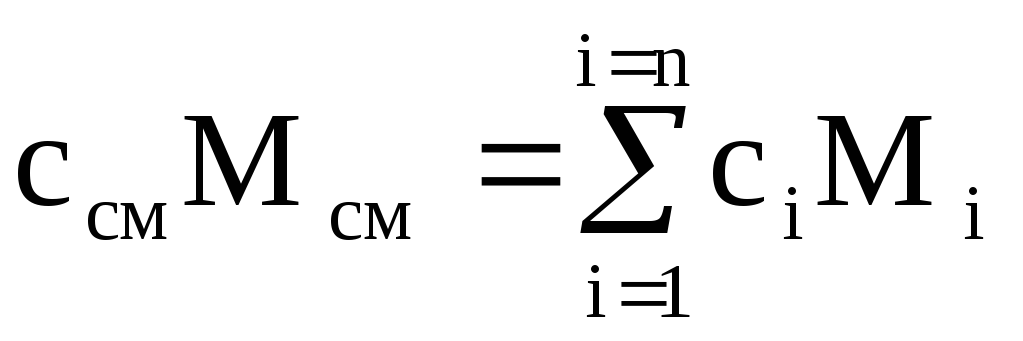 ,
,
where c i and c cm are the mass heat capacities of the i-th component and mixture.
Dividing the last expression by M cm, we obtain the calculation formula for the mass heat capacity of the mixture:
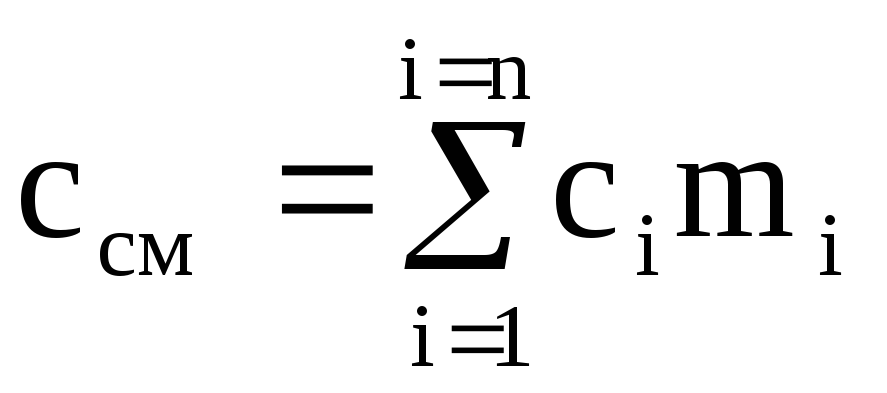 ,
(1.40)
,
(1.40)
where m i is the mass fraction of the i-th component.
Arguing similarly, we find the volumetric heat capacity c¢ cm and the molar heat capacity c m cm of the mixture:
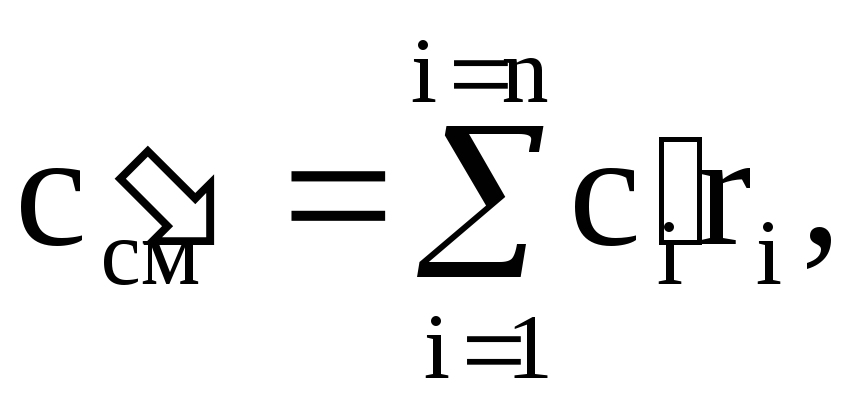 (1.41)
(1.41)
where c¢ i - volumetric heat capacity of the i-th component, r i - volume fraction of the i-th component,
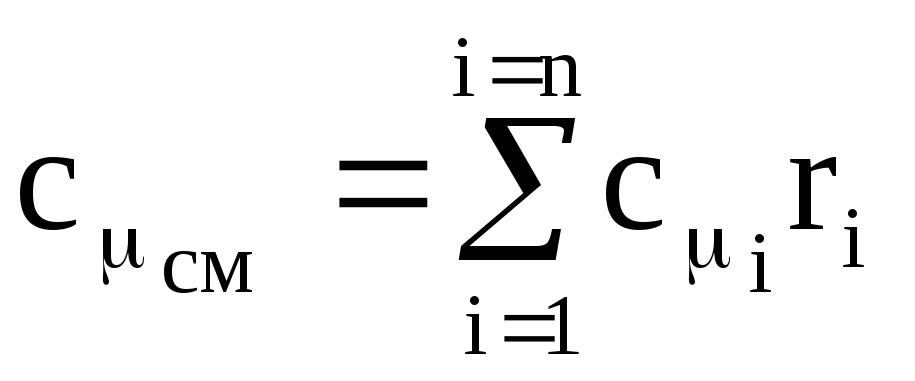 ,
(1.42)
,
(1.42)
where c m i is the molar heat capacity of the i-th component,
r i - mole (volume) fraction of the i-th component.
In heat engines (machines), the working fluid is a mixture of various gases. If the components of the mixture do not enter into chemical reactions with each other, and each component obeys the Claiperon equation of state, then such a mixture is considered as an ideal gas.
To calculate the mixture, it is necessary to determine μ cm - the average molar mass and R c m - the specific gas constant of the mixture. To determine them, it is necessary to know the composition of the mixture, that is, which components and in what quantities form this mixture, what parameters each component included in the mixture has.
Each component of the mixture behaves as if there were no other gases in the mixture, occupies the entire available volume in which the mixture is located, follows its own equation of state and exerts its so-called partial pressure on the walls, while the temperature of all components of the mixture is the same and equal to mixture temperature.
According to Dalton's law, the pressure of the mixture P is equal to the sum of the partial pressures of the individual components included in the mixture:
where n is the number of mixture components.
According to Amag's law, the volume of the mixture V is equal to the sum of the partial volumes of the individual components included in the mixture at the temperature and pressure of the mixture:
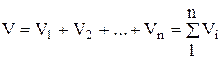 , (1.21)
, (1.21)
where - partial volume, m 3; V- volume of the mixture, m 3
The composition of the mixture is given by volume (molar) or mass fractions.
Volume fraction of the i-th component is the ratio of the partial volume of the component to the volume of the mixture, i.e., then the sum of the volume fractions of the components of the mixture is 1, i.e. . If the value is given in %, then their sum = 100%.
Molar fraction of the i-th component n i is the ratio of the number of kilomoles of the component N i to the number of kilomoles of the mixture N, i.e., where ![]() , , i.e. the number of kilomoles of each component and the mixture as a whole is equal to the ratio of the corresponding component and the mixture as a whole to the volume occupied by one kilomol.
, , i.e. the number of kilomoles of each component and the mixture as a whole is equal to the ratio of the corresponding component and the mixture as a whole to the volume occupied by one kilomol.
Considering that an ideal gas under the same conditions has the same volume of kilomole, then after substitution we get:, i.e. for ideal gases, the molar and volume fractions are numerically equal.
Mass fraction of the i-th component is the ratio of the mass of the component to the mass of the mixture: , it follows that the mass of the mixture is equal to the sum of the masses of the components, and also the sum of the mass fractions of the components is equal to 1 (or 100%).
Conversion of volume fractions to mass fractions and vice versa is based on the following ratios:
![]() ,
,
where ρ = μ / 22.4, kg / m 3.
Whence it follows that the mass fraction of the i-th component will be determined from the relation:
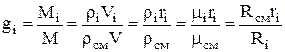 ,
,
where is the density of the mixture, kg / m 3, is the volume fraction of the i-th component.
In the future, it can be determined through volume fractions.
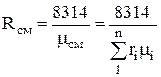 .
.
Density mixtures for volume fractions is determined from the ratio
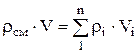 , where
, where 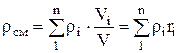 , (1.22)
, (1.22)
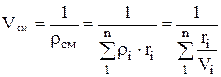 .
.
Partial pressure is determined by the formulas:
![]() or
or 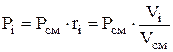 (1.23)
(1.23)
The equations of state of the components and the mixture as a whole have the form:
![]() ;
;
![]() ,
,
whence, after transformations, we obtain for massive shares
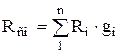 ,
, 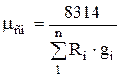 . (1.24)
. (1.24)
Density and specific volume of the mixture for massive share:
; 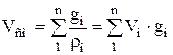 . (1.25)
. (1.25)
To calculate partial pressures, the formula is used:
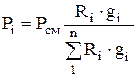 . (1.26)
. (1.26)
The conversion of mass fractions into volume fractions is carried out according to the formula:
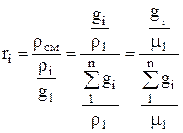 .
.
When determining the heat capacity of a mixture of gases, it is assumed that in order to heat (cool) a gas mixture, it is necessary to heat (cool) each of the components of the mixture
where Q i =M i c i ∆t is the heat spent on changing the temperature of the i-th component of the mixture, c i is the mass heat capacity of the i-th component of the mixture.
The heat capacity of the mixture is determined from the ratio (if the mixture is given by mass fractions)
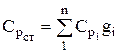 , similarly
, similarly 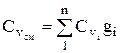 . (1.28)
. (1.28)
The molar and volumetric heat capacities for a mixture given by volume fractions are determined by
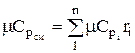 ;
; 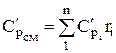 ;
;
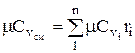 ;
; 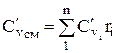
Example 1.5 Dry air by mass consists of g O2 \u003d 23.3% oxygen and g N 2 \u003d 76.6% nitrogen. Determine the composition of air by volume (r O2 and r N 2) and the gas constant of the mixture.
Solution.
1. From Table 1 we find kg/kmol and kg/kmol
2. Determine the volume fractions of oxygen and nitrogen:
1. The gas constant of air (mixture) is determined by the formula:
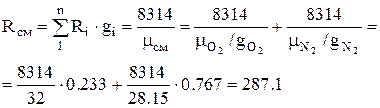 , J/kg K
, J/kg K
Example 1.6. Determine the amount of heat required to heat a gas mixture with a mass of M = 2 kg at P = const, consisting in% by weight: , , , , when the temperature changes from t 1 =900 ° C to t 2 = 1200 ° C.
Solution:
1. Determine the average mass heat capacity of the components that make up the gas mixture at P=const and t 1 =900 o C (from P2):
1.0258 kJ/kg K; =1.1045 kJ/kg K;
1.1078 kJ/kg K; =2.1097 kJ/kg K;
2. We determine the average mass heat capacity of the components that make up the gas mixture at P=const and t 1 =1200 o C (from P2):
1.0509 kJ/kg K; =1.153 kJ/kg K;
1.1359 kJ/kg K; =2.2106 kJ/kg K;
3. We determine the average mass heat capacity of the mixture for the temperature range: t 2 \u003d 1200 ° C and t 1 \u003d 900 ° C:
4. The amount of heat for heating 2 kg of the mixture at P=const:
First law of thermodynamics establishes a quantitative relationship between the change in the internal energy of the system and the mechanical work performed against the forces of external pressure of the environment as a result of the supply of heat to the working fluid.
For a closed thermodynamic system, the equation of the first law has the form
The heat imparted to the working fluid (or system) is used to increase its internal energy (dU) due to an increase in body temperature, and to perform external work (dL) due to the expansion of the working fluid and an increase in its volume.
The first law can be written as dH=dq+VdP=dq-dL 0 ,
where dL 0 \u003d VdP - the elementary work of pressure change is called useful external (technical) work.
dU is the change in the internal energy of the working fluid (system), which includes the energy of the thermal motion of molecules (translational, rotational and vibrational) and the potential energy of the interaction of molecules.
Since the transition of the system from one state to another occurs as a result of heat supply, therefore, the working fluid heats up and its temperature rises by dT and the volume increases by dV.
An increase in body temperature causes an increase in the kinetic energy of its particles, and an increase in body volume leads to a change in the potential energy of particles. As a result, the internal energy of the body increases by dU, so the internal energy U is a function of the state of the body and can be represented as a function of two independent parameters U=f 1 (P,V); U=f 2 (P,T), U=f 3 (υ,T). The change in internal energy in a thermodynamic process is determined only by the initial (U 1) and final (U 2) states, i.e.
In differential form, the change in internal energy is written
a) as a function of specific volume and temperature
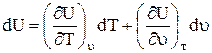
b) as a function of temperature, since , That
For practical calculations, in which it is necessary to take into account the change in C v with temperature, there are empirical formulas and tables of specific internal energy (often molar). For ideal gases, the molar internal energy of the mixture U m is determined by the formula
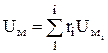 , J/kmol
, J/kmol
For a mixture given by mass fractions . Thus internal energy There is property of the system and characterizes the state of the system.
Enthalpy is the thermal state function introduced by Kamerling-Onnes, (winner Nobel Prize, 1913), which is the sum of the internal energy of the system U and the product of the pressure of the system P and its volume V.
Since the quantities included in it are state functions, therefore H is also a state function, i.e. H \u003d f 1 (P, V); H=f 2 (V,T); H=f 3 (P, T).
The change in enthalpy dH in any thermodynamic process is determined by the initial H 1 and final H 2 states and does not depend on the nature of the process. If the system contains 1 kg of a substance, then the specific enthalpy, J/kg, is applied.
For an ideal gas, the differential equation has the form
accordingly, the specific enthalpy is determined by the formula
The equation of the first law of thermodynamics is dq=dU+Pdυ, when the only type of work is the expansion work Pdυ=d(Pυ)-υdP, then dq=d(U+Pυ)-υdP, whence
Practical work№ 2
Topic: Heat capacity, enthalpy, mixtures of ideal gases, internal energy, work, thermodynamic processes.
The purpose of the work: Consolidation of knowledge gained during theoretical training, acquisition of skills in the implementation of heat engineering calculations.
I. Basic definitions, formulas and equations
1. Mixtures of ideal gases
A gas mixture is a mechanical mixture of several gases that do not chemically interact with each other. Each of the gases in the mixture is called a gas component; behaves as if there were no other gases in the mixture, i.e. evenly distributed throughout the mixture. The pressure exerted by each gas of the mixture on the walls of the vessel is called partial pressure. The basic law for mixtures of ideal gases is Dalton's law, according to which the pressure of the mixture is equal to the sum of the partial pressures of the gases that form the mixture:
2. Internal energy
The internal energy of the body is a combination of the kinetic energy of the movement of the microparticles that make up the body, and their potential energy. interaction defined. forces of mutual attraction or repulsion. It is impossible to determine the absolute value of the internal energy, therefore, in thermodynamic calculations, it is not the absolute value of the internal energy that is calculated, but its change, i.e.
![]() or
or ![]()
where U 1 and U 2 - the internal energy of the initial and final state of the working fluid (gas);
u 1 and and 2 - beats. internal energy of the initial and final state of the working fluid.
It follows from this that the change in internal energy does not depend on the nature and path of the process, but is determined by the state of the working fluid at the beginning and end of the process of change.
A feature of an ideal gas is the absence of forces of molecular interactions in it, and hence the absence of internal potential energy, i.e. U n \u003d 0 and U „ \u003d 0. Therefore, the internal energy of an ideal gas:
U=U k =f(T) unu u=uk =f(T).
H. Gas work.
In thermodynamics, any change in the state of the working fluid as a result of the exchange of energy with environment called a process. In this case, the main parameters of the working body are changed:
The transformation of heat into mechanical work is associated with the process of changing the state of the working fluid. The processes of changing the state of a gas can be expansion and contraction processes. For an arbitrary mass of gas M (kg), the work is equal to:
L \u003d M l \u003d Mp (v 2 - v 1) \u003d, J
where l \u003d p (v 2 -v 1) J / kg is the work of 1 kg of gas or specific work.
4. Gas enthalpy,
Enthalpy is a parameter that characterizes the potential energy of the connection of the working fluid (gas) with the environment. Enthalpy and specific enthalpy:
I \u003d U + pV, J and i i \u003d and + pv, J / kg.
5. Heat capacity.
Specific heat capacity is the amount of heat that must be supplied to 1 kg of gas in order to heat it by 1 ° C in a given temperature range.
Specific heat capacity is mass, volumetric and kilomol. There is a connection between mass C, volume C and kilomol C heat capacities:
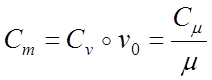 ;
; 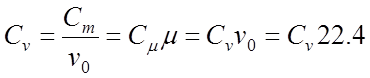
where Vo 22.4 m 3 / kmol - beats. volume of gas under normal conditions.
Mass ud. heat capacity of the gas mixture:
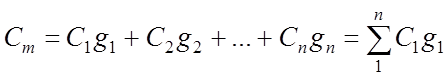
Volumetric specific heat gas mixture:
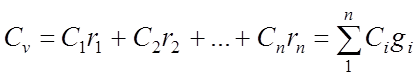
Kilomolar specific heat of the gas mixture:
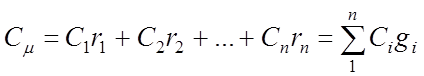
6. Equation for determining the amount of heat
The amount of heat given off or taken in by the working fluid (gas) can be determined by the equation:
Q \u003d M C m (t 2 -t 1), J or Q \u003d VC (t-t), J, where M and V are the weight or volume amount of gas, kg or m 3;
t u t - gas temperature at the end and at the beginning of the process ° С;
C and C - mass and volume average beats. heat capacity of gas
At t cp \u003d J / kgK or J / m 3 K
7. First law of thermodynamics
This law considers the interconversions of heat and mechanical work. According to this law, heat is converted into mechanical work and vice versa, mechanical work into heat in strictly equivalent quantities. The equivalence equation for heat and work has the form:
Taking into account the principle of equivalence of heat and work, the heat balance equation for an arbitrary mass of gas:
Q \u003d U + L and q \u003d u + l \u003d u -u + l
Problem solvingII
Task #1 (#1)
Atmospheric dry air has the following approximate mass composition: g 02 =23.2%, g N 2 =76.8%.
Determine the volumetric composition of air, its gas constant, apparent molecular weight, partial pressure of oxygen and nitrogen, if air is P = 101325 Pa using a barometer.
I determine the volumetric composition of air:
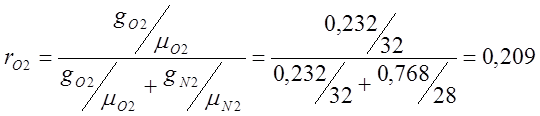 ;
;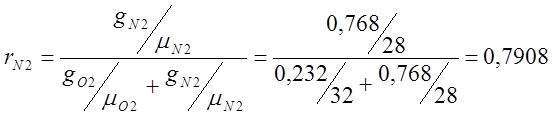 ;
;
where r is the mass fraction;
m is the relative molecular weight;
g is the volume fraction.
m air. =m O2 r O2 +m N2 r N2 = 32 0.209 + 28 0.7908=6.688+22.14=28.83;
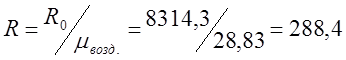
 ;
;
where R 0 is the gas constant.
I determine the partial pressures of various gases:
P O 2 \u003d P cm r O2 \u003d 101325 0.209 \u003d 21176.9 (Pa);
P N 2 \u003d P cm r N 2 \u003d 101325 0.7908 \u003d 80127.81 (Pa);
where P O 2 , P N 2 - partial pressure;
P cm is the pressure of the mixture.
Task #2 (#2)
The vessel is divided by a partition into 2 parts, the volumes of which are V 1 =1.5 m 3 and V 2 =1.0 m 3 . The first part of the volume V 1 contains CO 2 at P 1 =0.5 MPa and t 1 =30°C; the second part of the volume V 2 contains O 2 at P 2 =0.2 MPa and t 2 =57°C. Determine the mass and volume fractions of CO 2 and O 2, the apparent molecular weight of the mixture and its gas constant after the partition is removed and the mixing process is completed.
I determine individual gas constants:
To do this, I determine the relative molecular weight: m (CO 2) \u003d 32 + 12 \u003d 44; m(O 2)=32;
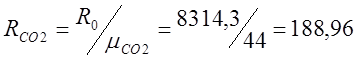
 ;
;
![]()
 ;
;
According to the characteristic equation of Klaiperon, I determine the masses of gases:
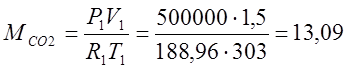 (kg);
(kg);
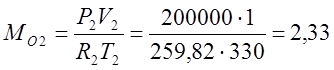 (kg);
(kg);
I determine the mass fractions:
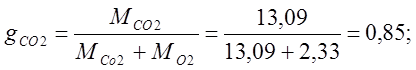
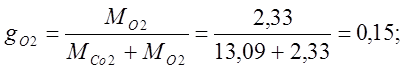
I determine the volume fractions:
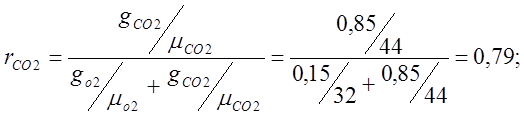
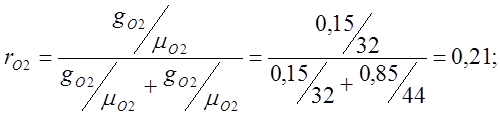
Determine the apparent molecular weight of air:
m air. \u003d m O2 r O 2 + m CO2 r CO2 \u003d 32 0.21 + 44 0.79 \u003d 6.72 + 34.74 \u003d 41.48;
I determine the individual gas constant for air (R):
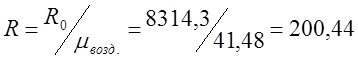
 ;
;
Task #3 (#6)
In a vessel with a volume of 300 l there is oxygen at a pressure P 1 \u003d 0.2 MPa and t 1 \u003d 20 0 C. How much heat must be supplied so that the oxygen temperature rises to t 2 \u003d 300 0 C? What pressure will be established in the vessel? For calculation, take the average volumetric specific heat of oxygen at n.o. C 02 \u003d 0.935 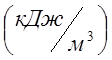
According to Charles's law, I determine the final pressure of the process:
; 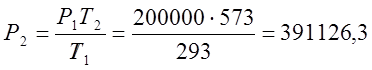 (Pa);
(Pa);
where P, T are gas parameters.
I determine the individual gas constant for oxygen (R):
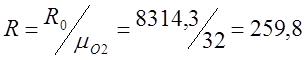
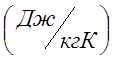 ;
;
Since the process is isochoric, I determine the amount of heat that needs to be supplied according to the appropriate formula: Q v \u003d M C cv (T 2 -T 1) for this, according to the Claiperon characteristic equation, I determine the mass of gas
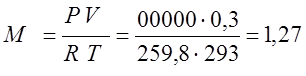 (kg); Q v \u003d M C cv (T 2 -T 1) \u003d 1.27 935 280 \u003d 332486 (J).
(kg); Q v \u003d M C cv (T 2 -T 1) \u003d 1.27 935 280 \u003d 332486 (J).
Task #4 (#7)
How much heat must be expended to heat 2m 3 of air at a constant overpressure P ex. \u003d 0.2 MPa from a temperature of 100 0 C to a temperature of 500 0 C. What work will air do in this case? For calculation, take: atmospheric pressure P at. \u003d 0.1 MPa, average mass isobaric heat capacity of air C pm \u003d 1.022 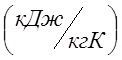 ; calculate the gas constant bearing in mind that the apparent molecular weight of air M air. =29.
; calculate the gas constant bearing in mind that the apparent molecular weight of air M air. =29.
I determine the individual gas constant for air:
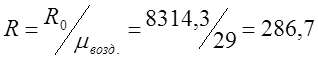
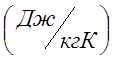 ;
;
The absolute pressure is equal to the sum of the excess and atmospheric P=P est. + P at. =0.1+0.2=0.3 MPa
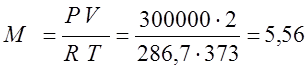 (kg);
(kg);
Since the process is isobaric, I determine Q and L according to the corresponding formulas:
according to the Gay-Lussac law, I determine the final volume:
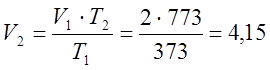 m 3;
m 3;
Q \u003d M C pm (T 2 -T 1) \u003d 5.56 1022 400 \u003d 2272928 (J);
L \u003d P (V 2 -V 1) \u003d 300000 2.15 \u003d 645000 (J).
Task #5 (#8)
There is air in the cylinder at a pressure P=0.5 MPa and a temperature t 1 =400 0 C. Heat is removed from the air at P=const so that at the end of the process the temperature t 2 =0 0 C is set. The volume of the cylinder in which air V 1 \u003d 400 l.
Determine the amount of heat removed, the final volume, the change in internal energy and the perfect work of compression C pm =1.028  .
.
Since the process is isobaric, then according to the Gay-Lussac law I determine the final volume:
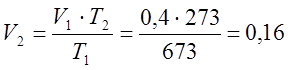 m 3;
m 3;
According to the characteristic equation of Klaiperon, I determine the mass of gas:
From the previous problem R=286.7 
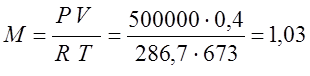 (kg);
(kg);
I determine the amount of heat that is released:
Q=M C pm (T 2 -T 1)=1.03 1028 (273-673)=-423536 (J);
I determine the amount of work spent:
L=P (V 2 -V 1)= 500,000 (0.16-0.4)=-120,000 (J);
From the equation by which the total amount is determined, I determine the change in the amount of internal energy:
![]() ; (J)
; (J)
Problem #6 (#9)
Air having a volume V 1 =0.02 m3 at a pressure P 1 =1.1 MPa and t 1 =25 s expands in a cylinder with a movable piston to a pressure P 2 =0.11 MPa. Find the final volume V 2, the final temperature t 2 , the work done by the air, and the heat supplied, if the expansion in the cylinder occurs:
a) isothermally
b) adiabatically with adiabatic exponent k=1.4
c) polytropic with polytropic index n=1.3
Isothermal process:
P 1 / P 2 \u003d V 2 / V 1
V 2 \u003d 0.02 1.1 / 0.11 \u003d 0.2M 3
Q=L=RMT 1 Ln(V 2 /V 1)=P 1 V 1 Ln(V 2 /V 1)=1.1 10 6 0.02Ln(0.2/0.02)=22000 J
adiabatic process:
V 1 / V 2 \u003d (P 2 / P 1) 1 / k
V 2 \u003d V 1 / (P 2 / P 1) 1 / k \u003d 0.02 / (0.11 / 1.1) 1 / 1.4 \u003d 0.1036M 3
T 2 /T 1 \u003d (P 2 /P 1) k-1 / k
T 2 \u003d (P 2 / P 1) k-1 / k T 1 \u003d (0.11 / 1.1) 1.4-1 / 1.4 298 \u003d 20.32k
C v \u003d 727.4 J / kg k
L \u003d 1 / k-1 (P 1 V 1 -P 2 V 2) \u003d (1 / 1.4-1) (1.1 10 6 0.02 -0.11 10 6 0, 1)=2.0275 10 6 J
Polytropic process:
V 1 / V 2 \u003d (P 2 / P 1) 1 / n
V 2 \u003d V 1 / (P 2 / P 1) 1 / n \u003d 0.02 / (0.11 / 1.1) 1 / 1.3 \u003d 0.118M 3
T 2 /T 1 \u003d (P 2 /P 1) n-1 / n
T 2 \u003d (P 2 / P 1) n-1 / n T 1 \u003d (0.11 / 1.1) 1.3-1 / 1.3 298 \u003d 175k
L \u003d 1 / n-1 (P 1 V 1 -P 2 V 2) \u003d (1 / (1.3-1)) (1.1 10 6 0.02 -0.11 10 6 0.118)=30000J
Q=(k-n/k-1) l M=((1.4-1.3)/(1.4-1)) 30000=7500J
Literature:
1. Energy, Moscow, 1975.
2. Litvin A.M. "Theoretical foundations of heat engineering", publishing house "Energy", Moscow, 1969.
3. Tugunov P.I., Samsonov A.A., “Fundamentals of heat engineering, heat engines and steam power facilities”, Nedra publishing house, Moscow, 1970.
4. Krutov V.I., "Heat engineering", publishing house "Engineering", Moscow, 1986.
Gas mixtures. Heat capacity of gases
Gas mixtures are understood as a mechanical mixture of several gases that do not chemically interact with each other. A mixture of ideal gases obeys all laws relating to ideal gases. The composition of the gas mixture is determined by the amount of each of the gases included in the mixture, and can be specified by mass or volume fractions:
where is the mass of the -th component, is the volume of the -th component, and and are the mass and volume of the entire mixture, respectively.
It's obvious that
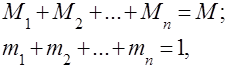
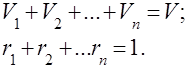
For the convenience of solving practical problems with gas mixtures, the concept of the apparent molecular weight of a gas mixture is introduced, which is the average mass of the actual molecular weights of the individual components of the mixture.
The equation of state for a mixture of gases has the form:
Gas mixtures are subject to the concept of the universal gas constant
The relationship between the pressure of a gas mixture and the partial pressure of the individual components included in the mixture is established by Dalton's law:
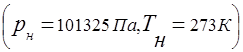
1 gas has a different mass depending on pressure and temperature. In this regard, the volumetric heat capacity is always referred to the mass of gas enclosed in 1 under normal conditions. In this case, the volume of 1 kmole of various gases is 22.4 / kmol, and the universal gas constant is . Depending on the method of heat supply to the gas, there are isobaric and isochoric heat capacities. The ratio of these quantities is called the adiabatic exponent
The heat capacities and are also related by the Mayer relation
The amount of heat that must be spent in the process of heating 1 kg of gas in the temperature range from to is determined by the formula:
where and are, respectively, the average heat capacities within 0°- and 0°-.
If (kg) or () gas is involved in the process, then
The heat capacity of the gas mixture should be determined by the formulas:
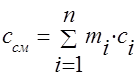
mass - ;
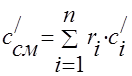
volumetric -;
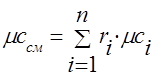
molar - .
To use the heat of gases that are products of fuel combustion in the boiler unit, air heaters of the air necessary for fuel combustion are installed in the gas ducts of the latter (Fig. 1). The gases leaving the boiler enter the air heater with a temperature and are cooled, giving off heat to the air, up to. In the gas duct of the boiler unit, under the influence of the operation of the smoke exhauster, a pressure is set slightly below atmospheric. The air in the air heater is heated from temperature to temperature.
flue gases
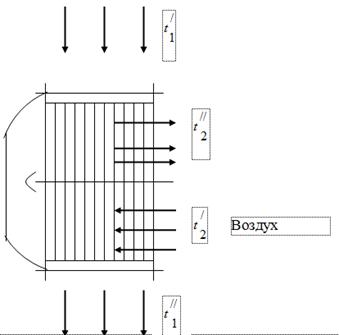
When testing the boiler unit, the following data were obtained:
The temperature of the gases at the inlet to the air heater, = 450 °C.
The temperature of the gases at the outlet of the air heater, = 150 ° C.
Air temperature at the inlet to the air heater, = 26 ° C.
Air temperature at the outlet of the air heater, = 260 ° C.
Volumetric composition of flue gases - = 11.5%; = 6.5%; = 17.2%; = 64.8%
The hourly consumption of gases at is 50 ·/h.
Vacuum in the gas duct - 15 mm of water. Art.
Barometric pressure 760 mm Hg. Art.
Define:
apparent molecular weight of flue gases;
flue gas gas constant;
weight (mass) fractions of individual components that make up the flue gases;
partial pressures of components:
hourly air flow.
Assume that all the heat given off by the gas is taken up by the air.
The dependence of heat capacity on temperature is considered to be curvilinear.
1. Apparent molecular weight of flue gases
0.115 44 + 0.065 18 + 0.172 32 + 0.648 28 =
5,06 + 1,17 + 5,504 + 18,144 = 29,878
2. Flue gas gas constant
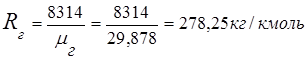
3. Mass fractions gas components
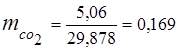
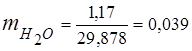
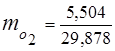
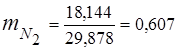
4. Partial pressures of the components
The results of the calculation will be entered in the table
|
Options |
gas mixture |
|||
|
|
||||
|
|
||||
|
|
5. Hourly air consumption
Air consumption is determined from the heat balance equation of the air heater
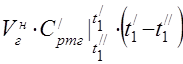
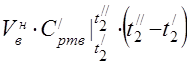
average molar heat capacity at 450 °C
The average specific heat capacity of the components at in the temperature range 0 ... 450 ° C.

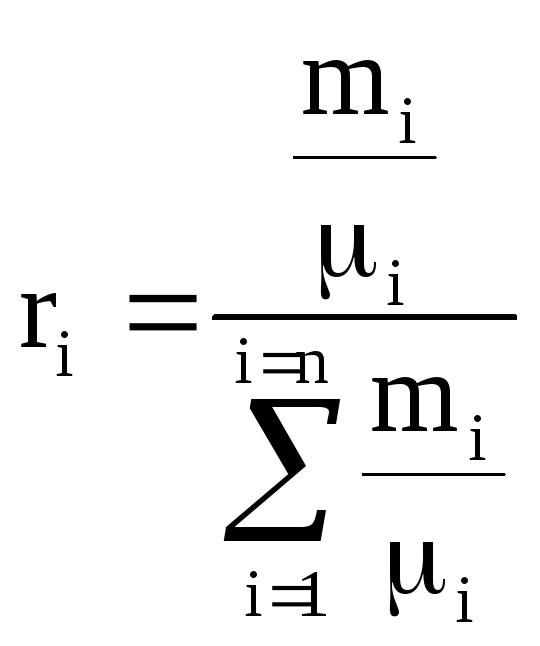
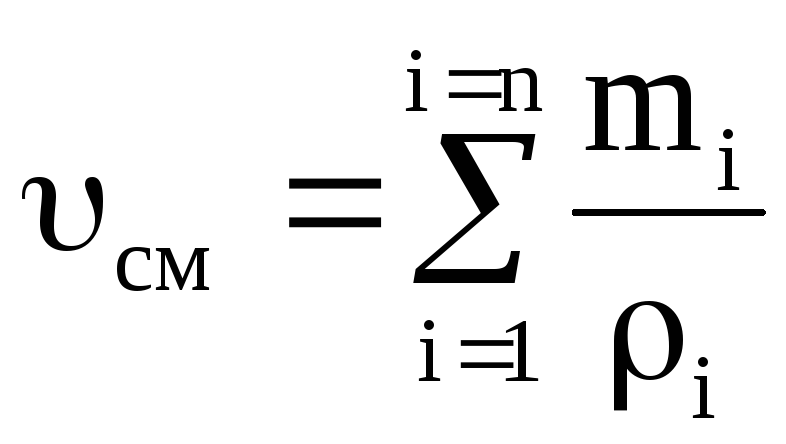
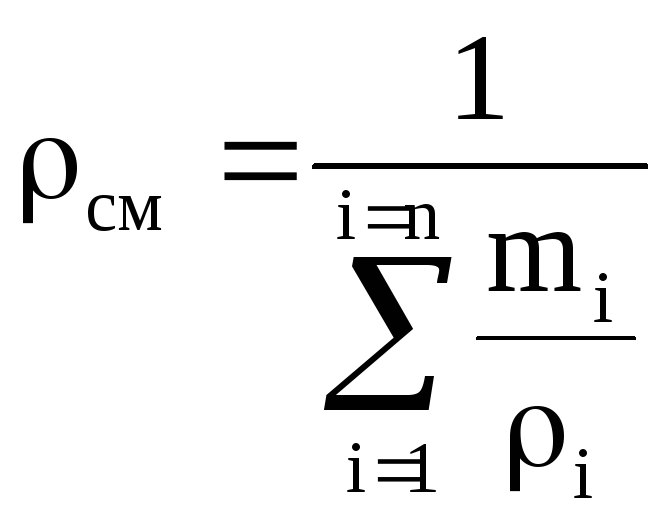
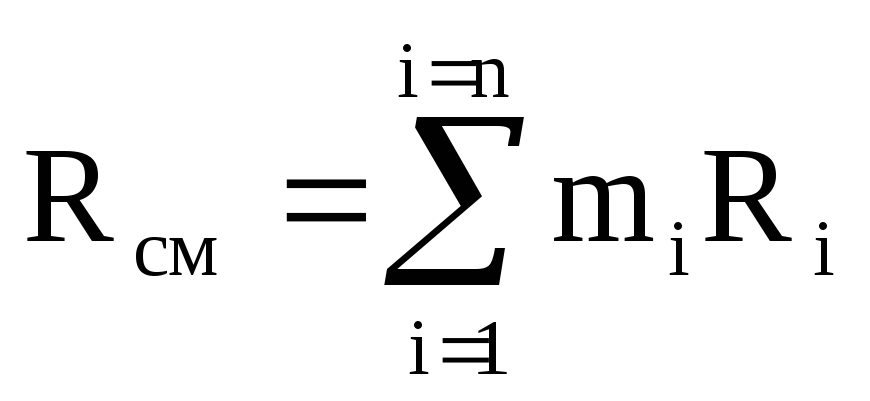

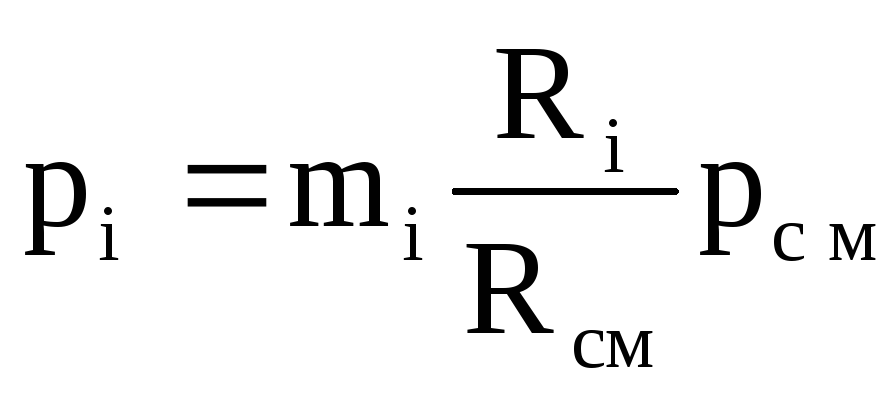
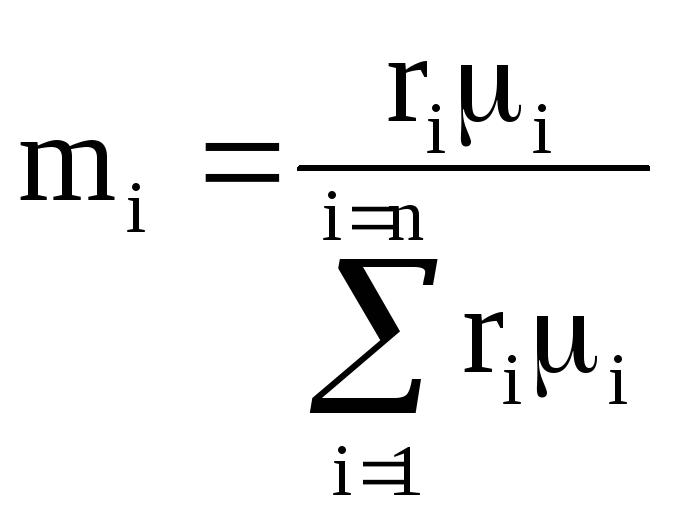
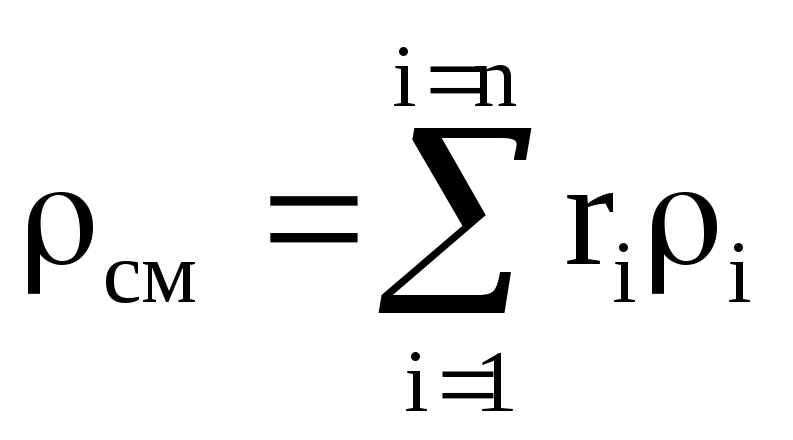
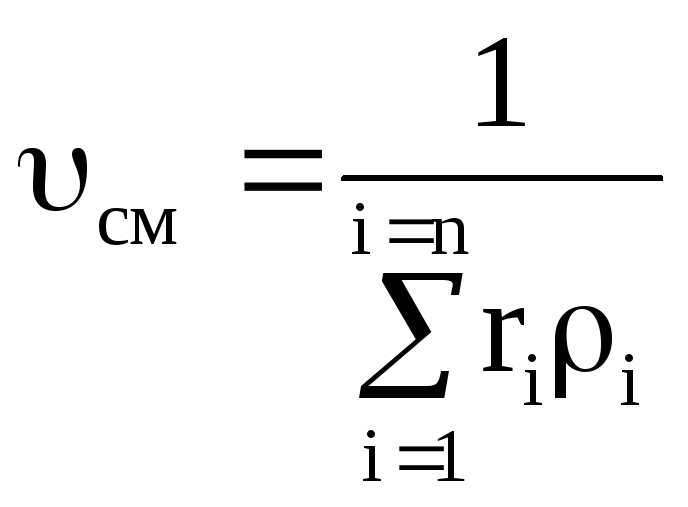
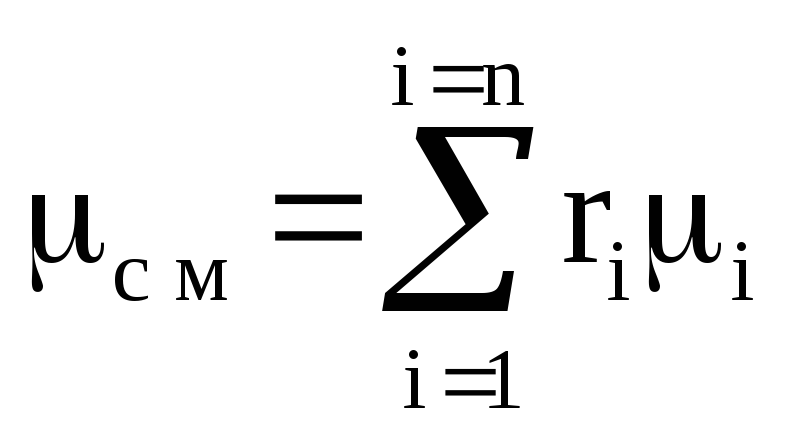
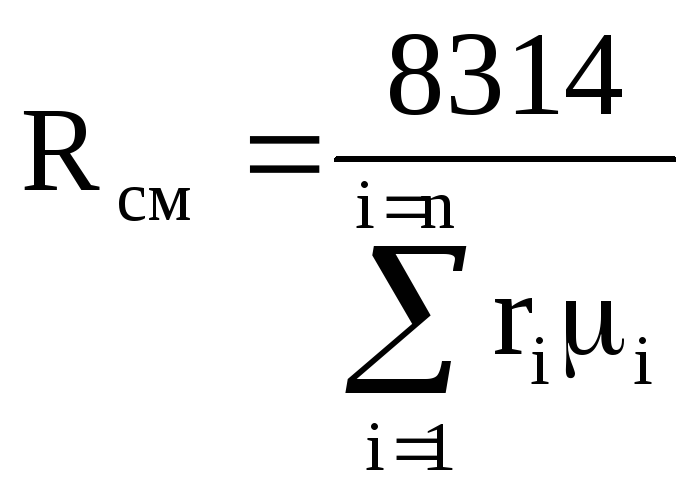
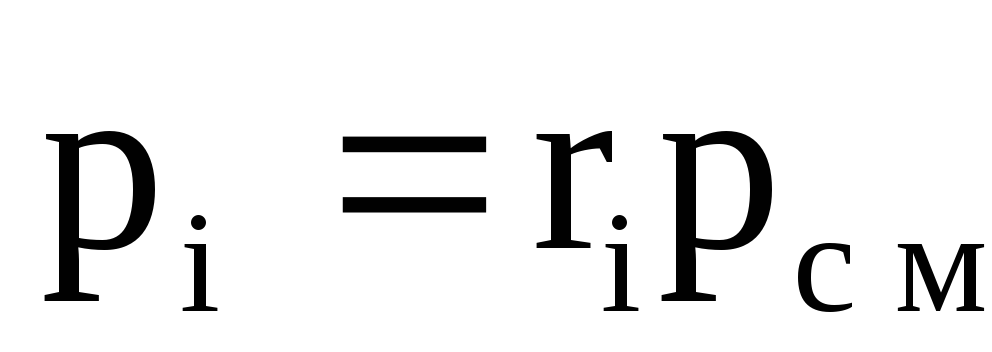
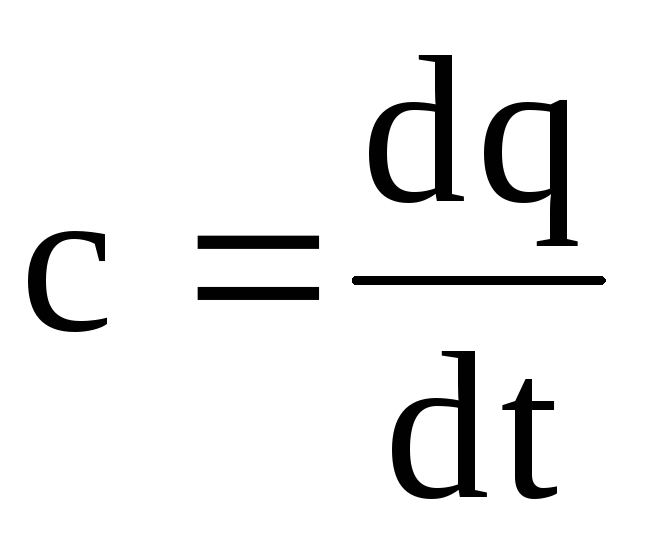 .
(1.28)
.
(1.28)