Specific heat capacity in the solid state. Tag Archives: heat capacity
The principles for calculating the heat capacity of metal utensils are applicable to batteries and bathtubs.
The cast iron battery cools down longer.
Once again, I would like to draw your attention to the fact that the rate of cooling of an object directly depends on the mass and specific heat of the material from which it is made. Do not confuse heat capacity and thermal conductivity!
A cast iron battery is three times heavier than an aluminum one. Therefore, it has higher heat capacity 2.5 times.
The question is often asked: why do cast iron batteries cool down longer than steel ones?
And specific heat capacities - 540 J / (kg * K) for cast iron and 460 J / (kg * K) for steel - differ relatively little (15%). And the whole secret - to a large extent - lies in the significantly larger mass of cast-iron batteries.
Battery section weight:
If we compare two batteries of the same mass - made of steel and cast iron - then at the same heating temperature, the cast-iron battery will retain heat by 15% more.
Cast iron tub retains heat.
Cast iron bath:
Steel bath:
That is, the amount of heat released during cooling by 1 degree in a cast-iron bath is 2.5 times greater than in a steel bath (in our example).
Heat capacity of bath water:
From what it follows, the temperature hot water(40 degrees) poured into a bath at room temperature (20 degrees) will drop 1 degree for a steel bath and 2.5 degrees for a cast iron bath.
Metal utensils through the eyes of a physicist
Returning to the topic of metal utensils, I will show the physics of processes in numbers.
Thermal conductivity.
Thermal conductivity is numerically equal to the amount of heat (J) passing through a unit area (sq.m) per unit time (sec) at a unit temperature gradient.
Thermal conductivity coefficients from the reference book:
Conclusion: cast iron distributes heat slowly. In other words, meat in a cast iron pan will not burn (including) due to a more even distribution of heat.
The situation is similar in cooking barbecue in nature. Cooking meat on coals allows you to bake the pieces. Cooking over an open fire simply broils the outside of the cuts of meat while leaving the insides raw.
Heat capacity.
The heat capacity is numerically equal to the amount of heat (J) that must be transferred in order to change its temperature by one unit (K).
Specific heat.
Specific heat capacity - the amount of heat (J) that must be transferred to a unit mass of a substance (kg) in order for its temperature to change by a unit temperature (K).
In other words, in order to calculate the heat capacity of a metal dish - how much thermal energy will be in a dish heated to the desired temperature - it is necessary to multiply the mass of the dish (kg) by the specific heat capacity of the metal (J / (kg * K)) from which it is made.
Specific heat values from the handbook:
Specific heat capacity is an important parameter that determines the characteristics of steel. It shows the amount of heat that needs to be expended to heat a kilogram of alloy by 1 degree. The heat capacity is influenced by different features of steel, which is especially important when
Under specific heat Steel refers to the amount of heat required to increase the temperature of one kilogram of a substance by exactly one degree. Both the Celsius and Kelvin scales can be used equally.
The heat capacity is influenced by many factors:
- state of aggregation of the heated substance;
- Atmosphere pressure;
- heating method;
- steel type.
In particular, high-alloy steels contain large amounts of carbon and are refractory. Accordingly, in order to heat up by one degree, more heat is needed than the standard 460 J / (kg * K). Low alloy steels heat up faster and easier. The maximum amount of heat and energy is needed to heat refractory materials with anti-corrosion treatment.
The calculation of heat capacity is made for each specific case. It must also be taken into account that with an increase in the temperature of the heated substance, its heat capacity changes.
Specific heat capacity is important when carrying out induction hardening or tempering of parts made of steel, cast iron, composite materials. When the product temperature rises by a certain number of degrees, phase changes occur in the structure, and, accordingly, the specific heat capacity also changes. Further heating will require more/smaller volumes of heat.
The specific heat capacity characterizes not only the process of heating steel or composite materials, but also their cooling. Each material, when cooled, gives off a certain amount of heat and / or energy. Specific heat capacity allows you to calculate how much heat will be obtained when one kilogram of metal cools by one degree. The heat transfer is affected by the area of the cooled material, the presence / absence of additional ventilation.
How is the specific heat capacity calculated?
Counting specific heat more often on the Kelvin scale. But thanks only to the difference in the reference point, the indicator can be converted to degrees Celsius.
The specific heat parameter determines the amount of fuel needed to heat the part to a given point. This depends on the type and grade of steel. A high alloy alloy has a higher parameter value at the same temperature. Low alloy and carbon steels - less.
Example:
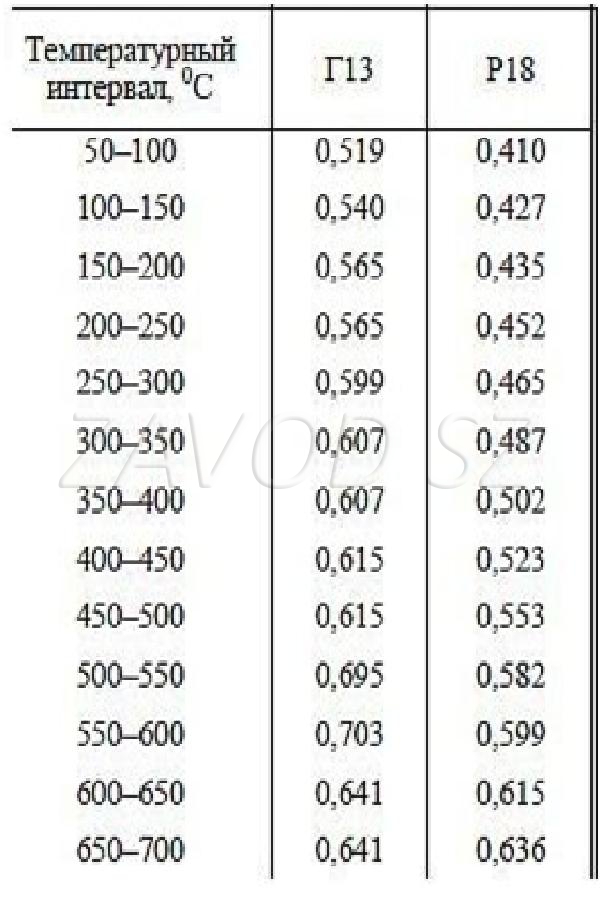
For comparison, G13 steel has a heat capacity of 0.520 kJ / (kg * deg) at a temperature of 100 ° C. This alloy is highly alloyed, that is, it contains more chromium, nickel, silicon and other additional elements. Carbon steel grade 20 at a similar temperature has a specific heat capacity of 0.460 kJ / (kg * deg).
Thus, the specific heat capacity depends not only on temperature, but also on the type of steel. High-alloy steels are less resistant to cracking and less weldable. The refractoriness of such materials is increased. These indicators directly affect which are made from different grades of steel. Stability, lightness, strength are the most important criteria that are determined by the quality of such an alloy.
In the tables, one can observe the indicators of the specific heat capacity of high-alloy steels G13 and R18, as well as a number of low-alloy alloys. Temperature ranges - 50:650оС.
Cast iron is a combination of iron and carbon. Among the main properties are the mass, shape, volume and placement of graphite impurities. In a state of thermodynamic equilibrium, the structure of iron-carbon alloys can be described by a diagram. During the modification of the composition changes:
Eutectic temperature (o C) T \u003d 1135 + 5 * Si - 35 * P - 2 * Mn + 4 * Cr;
saturation of the eutectic with carbon (%) С = 4.3 - 0.3*(Si+P) - 0.04*Ni - 0.07*Cr;
eutectoid transformation temperature (o C) T = 723 + 20*Si + 8*Cr - 30*Ni - 10*Cu - 20*Mn;
saturation of the eutectoid with carbon (%) C = 0.8 - 0.15 * Si - 0.8 * Ni - 0.05 * (Cr + Mn).
The placement of critical points depends on the degree of heating - in the case of cooling, they move slightly down. The most accurate simple formulas have been established for the overwhelming number , which does not contain alloying components:
Saturation of eutectic with carbon C = 4.3 – 0.3*(Si+P);
saturation of the eutectoid with carbon C = 0.8 - 0.15 * Si.
The effect of the compounds on the structure can be seen in Table 1. The coefficients that determine the conditional graphitising effect can be taken into account only in the presence of (C) (about 3%) and silicon (Si) (about 2%).
Table 1. Approximate influence of elements on the structure of cast iron
|
Elements |
Relative graphitising action |
||||
|
On the main metal mass |
On graphite |
When solidified |
in solid state |
||
|
Perlite reduction |
|||||
|
Perlite reduction |
Increasing the number and consolidation |
+0.2 to +0.5 |
|||
|
Manganese |
over 0.8 |
Grinding perlite |
Weak crushing |
-0.2 to +0.5 |
|
|
Formation of manganese sulfide |
Same, but less |
-0.2 to +0.5 |
|||
|
Sulfide formation |
Quantity reduction |
||||
|
Grinding perlite |
Increasing the amount and weak grinding |
+4 to -0.2 |
|||
|
Grinding perlite |
Reducing the amount and weak grinding |
-1.2 to -3.0 |
|||
|
Does not affect |
Not installed |
+0.3 to -0.2 |
|||
|
Molybdenum |
Grinding perlite. Needle structure formation |
-0.5 to -1.5 |
|||
|
Grinding perlite |
Quantity reduction. Significant crushing |
||||
|
Aluminum |
Perlite reduction |
Increasing the number and consolidation |
|||
|
Cerium and magnesium |
Spheroidinization |
||||
Physical and mechanical properties
The most important indicators of the physical and mechanical properties of the microstructure of cast iron can be found in Table. 2, physical properties - in table. 3. Specified in the 3rd table. the specific gravity can fluctuate greatly due to fluctuations in the volume of combined carbon and changes in the number of pores. The specific gravity of cast iron at the time of its melting is 7 ± 0.1 g / cm 3. When adding various simple impurities, it decreases. The coefficient of thermal expansion indicated in table 3 is influenced by the structure of cast iron.
A strong irreversible increase in volume occurs in the case of a change in temperature, at which an equilibrium phase transition occurs in a physical system. The indicator can reach 30%, but often it does not exceed 3% when heated to 500 ° C. The increase in volume is facilitated by the components that form graphites, and the components that form carbides interfere, as well as the coating of cast iron by enameling, metallization and galvanization.
Table 2. Physical and mechanical properties of the structural components of unalloyed cast iron
|
Structural component |
Specific gravity G/cm 3 |
Thermal linear expansion coefficient a * 10 - in 1 / o C at temperatures of 20 -100 o C |
Heat capacity in cal / G * o C at a temperature in o C |
Thermal conductivity in cal / cm * sec about C |
Electrical resistance in µΩ 9 cm |
Tensile strength σ in in kg / mm 2 |
Elongation σ in % |
Hardness HB |
||||
|
austenite |
||||||||||||
|
Cementite |
||||||||||||
Thermal properties
The heat capacity index of cast iron of a particular composition can be established according to the mixing law using the information given in table 2. It can be equal to 0.00018 kcal / (g o C) when the temperature overcomes the phase transition threshold, up to the melting temperature. After overcoming the melting point - 0.00023 ± 0.00003 kcal/(g o C). The thermal effect during solidification is 0.055 ± 0.005 kcal/g, and in the case of eutectoid decomposition of austenite, it is determined by the volume of pearlite included, and can reach 0.0215 ± 0.0015 kcal/g at a eutectoid concentration of 0.8% C st.
The heat capacity per unit volume of this substance can be used for enlarged calculations: for cast iron in the solid state - approximately 0.001 kcal / cm 3 o C, and in the liquid state - 0.0015 kcal / cm 3 o C.
Thermal conductivity cannot be established by the law of mixing; indicated in the table. 2, its indicators for elements, with an increase in their sizes in dispersed systems, decrease. Typical indicators of thermal conductivity are shown in table. 3. The role of the components included in the cast iron in changing the thermal conductivity can be seen in the deviations in the level of graphitization. The thermal conductivity of iron decreases with an increase in the volume of various additives included in it.
Cast iron in the molten state has a thermal conductivity of about 0.04 cal/cm s o C.
Using enlarged calculations, the thermal conductivity of cast iron in the solid state is equated to its thermal conductivity, and in the molten state - to 0.3 mm 2 / s.
Table 3. Typical physical properties cast iron
|
cast iron type |
Note, with increasing temperature: "+" - increases; "-" - goes down |
|||
|
Specific gravity G/cm 3 |
||||
|
Thermal linear expansion coefficient a 10 - in 1 / o C, at temperatures of 20-100 o C |
||||
|
Actual shrinkage in % |
||||
|
Thermal conductivity in cal/cm sec o C |
||||
|
Dynamic viscosity at liquidus temperature dyn sec/cm 2 |
||||
|
Surface tension in dynes / cm 2 |
||||
|
Electrical resistance in Mk ohm cm |
||||
|
Heat capacity in cal/G o C |
||||
|
Coercive force in e |
||||
|
Remanent magnetism in gs |
Hydrodynamic properties
Absolute viscosity indicators can be found in table. 4. Viscosity tends to decrease with an increase in the share, as well as in the case of a decrease in the part of sulfur and additives of non-metallic origin, due to temperature indicators.
The decrease in viscosity and the ratio of the absolute temperatures of the experiment and the moment of solidification are in direct proportion. During the transition of the temperature of the beginning of solidification, the viscosity increases rapidly.
Data on the surface tension of cast iron for coarse-grained calculations can be taken from Table 3. It increases with a decrease in the proportion of carbon and changes rapidly when components of non-metallic origin are added to the composition.
To determine the electrical characteristics, you can use the Kurnakov law. Approximate impurity values can be found in Table. 2, and, specifically cast iron - in table. 3. Effect of incoming components on electrical resistance solid conditionally can be placed in the following sequence, in descending order: (Si), manganese (Mn), (Cr), (Ni), (Co).
Table 4. Cast iron viscosity coefficients
|
Temperature in o C |
Viscosity coefficient in (dyne sec / cm 2) cast iron with carbon content in% |
||||||
|
Cast iron turns white |
|||||||
|
Cast iron turns gray |
|||||||
Mechanical properties
Statistical characteristics. The tensile strength (mechanical stress threshold) of cast iron can be calculated in a qualitative way, based on its structure according to the indicators indicated in Table 2. The strength of the components included in the structure of cast iron increases with an increase in their weighted sizes in dispersed systems. The structure, number, volume and location of graphite components have the greatest influence on the threshold of mechanical stress; the structure of the total mass of the metal is not so important.
The maximum decrease in strength is observed when placing chain-like graphite components, which make the metal structure not so continuous. The maximum strength indicators of the metal are given by the spheroidal structure of graphite. With an increase in the temperature of the test process, the threshold of mechanical stress, by and large, does not change up to 400 ° C (in the range from 100 to 200 ° C, the strength decreases slightly, within 10 - 15%). After overcoming the indicator of 400 ° C, a constant loss of mechanical stress threshold indicators is recorded.
Plasticity characteristics are determined by the structure of the total mass of the metal (according to the indicators given in Table 2), but even more significantly - by the form of graphite impurities. If the shape is spheroidal, then the elongation can reach up to 30%. In gray cast iron, such an elongation almost never reaches even a tenth of a percent. Elongations in calcined gray cast iron (ferritic) can be approximately 1.5%.
Elasticity is determined, by and large, by the graphite structure. It does not change in the process of thermal action on cast iron, if no changes were made to the form of graphite impurities. Bending tests show the proportion of elastic deformations equal to 50 - 80% of the total deformation.
The creep of cast iron should not be confused with the case of growth (an irreversible increase in its volume). Cast iron, which does not contain alloying components, when heated above 550°C, is characterized by permanent deformations, depending on its growth, prevailing over the deformations acceptable in the determination of creep. If its speed is 0.00001% per hour, then for 1 thousand hours at a load of 3 kg / mm 2, gray cast iron without alloying components exhibits stability at temperatures within 400 ° C, and cast iron containing alloying components - up to 500 ° C. An increase in creep resistance can be achieved with austenitic cast iron, as well as cast iron with the addition of molybdenum or with an increased presence of nickel and chromium.
If there are additives in the form of graphite in cast iron, then its modulus of elasticity will be only conditional. This indicator is not determined by the structure of the bulk of the metal, and is characterized by the proportion of graphite additives and their structure: it decreases with an increase in the proportion of graphite additives and with a decrease in their similarity to the globular structure.
Impact strength is not a completely accurate characteristic of dynamic qualities. It grows with an increase in ferrite inclusions, in the case of a decrease in graphite inclusions, and also when the structure of the graphite component is as similar as possible to a spherical one. With an uneven period of loading, the fatigue limit reaches a maximum due to the increase in stresses that occur in the direction of application of the load. The fatigue limit increases with an increase in the mechanical stress threshold and load repeatability.
Technological properties
Fluidity is determined by metallic properties and structure. It often depends on the length of the casting being filled, and increases with a decrease in viscosity, an increase in overheating (however, fluidity is most affected by overheating above the pour point), a decrease in the solidification interval, and is determined by the latent heat of fusion and heat capacity, expressed by volume.
Chemical properties
The degree of resistance to oxidation is due to the structure of cast iron and environment (chemical composition, temperature and its course). The elements that make up cast iron have an electrode potential. By decreasing this value, they can be arranged in the following sequence: graphite (iron carbide), double or triple phosphide eutectic - oxyfer.
The voltage between graphite and oxyfer (ferrite) is 0.56 volts. The degree of resistance to corrosion decreases with a corresponding increase in the level of dispersion of the constituent components. However, lowering the fineness level of the iron carbide too much lowers the degree of resistance to oxidation. Alloy components affect the ability of cast iron to resist oxidation along with their effect on the structural composition. Excessive resistance to oxidative processes is noted in cast iron castings with a preserved crust after.
α , specific heat capacity With and thermal conductivity λ depend on the composition and structure of cast iron, as well as on temperature. Therefore, their values are given in the appropriate temperature range. With increasing temperature values α and With usually increase and λ decreases (Table 1).
Linear expansion coefficient α and specific heat capacity c real inhomogeneous structures, including cast iron, can be determined by the mixing rule:
where x 1, x 2, ..., x n - α
or c structural components (Table 2);
a 1 , a 2 , ..., a n- their quantitative content.
Thermal conductivity of alloys and mixtures, in contrast to the coefficient α and heat capacity c cannot be determined by the mixing rule. The influence of individual elements on thermal conductivity can be established only approximately by calculation.
Per coefficient α and specific heat capacity With mainly affects the composition of cast iron, and the thermal conductivity λ - the degree of graphitization, the dispersion of the structure, non-metallic inclusions, etc.
The coefficient of linear expansion determines not only the changes in dimensions depending on temperature, but also the stresses formed in the castings. Decrease α is useful from these positions and facilitates the conditions for obtaining high-quality castings. But in the case of joint operation of cast iron parts with parts made of non-ferrous alloys or other materials with a higher coefficient of linear expansion, it is necessary to strive to increase the value α for cast iron.
The heat capacity and thermal conductivity are great importance for castings such as heating pipes, moulds, parts refrigeration units and engines internal combustion etc., since they determine the uniformity of temperature distribution in castings and the intensity of heat removal.
In table. 3 shows the thermophysical properties of cast irons of various groups.
| Cast iron | α 20 100 ∗10 6 , 1/°C | c 20 100 , J/(kg∗°C) | c 20 1000 , J/(kg∗°C) | λ 20 100 , W/(m∗°C) |
|---|---|---|---|---|
| Gray with lamellar graphite (GOST 1412-85): | MF10-MF18 | 10-11 | 502-544 | 586-628 | 46,0-54,4 |
| MF20-MF30 | 10-11 | 502-544 | 586-628 | 41,8-50,2 |
| MF35 | 11,5-12,0 | 502-544 | 628-670 | 37,6-46,0 |
| High strength (GOST 7293-85): | ||||
| HF 35-HF 45 | 11,5-12,5 | 460-502 | 586-628 | 37,6-46,0 |
| HF 60-HF 80 | 10-11 | 502-523 | 628-670 | 33,5-41,9 |
| HF 100 | 9-10 | 523-565 | 628-670 | 29,3-37,6 |
| Malleable (GOST 7769-82): | ||||
| KCh 30-6/KCh 37-12 | 10,5-11,0 | 460-511 | 586-628 | 54,4-62,8 |
| KCh 45-5/KCh 65-3 | 10,3-10,8 | 527-544 | 628-670 | 50,2-54,4 |
| Alloyed (GOST 7769-82) | ||||
| nickel ChN20D2Sh | 17-19 | — | 460-502 | 17,4 |
| with 35-37% Ni | 1,5-2,5 | — | — | — |
| chromic: | ||||
| CH16 | — | — | — | 32,5 *1 |
| CH22 | — | — | — | 25,5 *1 |
| CH28 | 9-10 | — | — | 17,4 *1 |
| CH32 | 9-10 | — | — | 19,8 *1 |
| siliceous: | ||||
| CHS5 | 14-17 *2 | — | — | 21,0 *3 |
| ChS15, ChS17 | 4,7 *1 | — | — | 10,5 |
| aluminum: | ||||
| ChYu22Sh | 17,5 *1 | — | — | 15,1-28,0 *3 |
| CHJ30 | 22-23 *2 | — | — | — |
| *1 Between 20-200°C. | ||||
| *2 Between 20-900°C. | ||||
| *3 Between 20-500°C. | ||||
Linear expansion coefficient α
Linear expansion coefficient α . The greatest impact on the coefficient α exerts carbon, especially in the bound state. One percent carbon corresponds to about 5 times large quantity cementite than graphite. Therefore, graphitising elements (Si, Al, Ti, Ni, Сu, etc.) increase, and anti-grafting (Cr, V, W, Mo, Mn, etc.) reduce the coefficient of linear expansion,
highest value α austenitic nickel cast irons differ, as well as ferritic aluminum cast irons of the cast iron and pyroferal type. Therefore, at a sufficiently high content Ni, Cu, Mn meaning α ; increases sharply. However, with the content Ni>20% α decreases: and reaches a minimum at 35-37% Ni. The shape of graphite significantly affects the coefficient of linear expansion only at low temperatures; α ductile iron with nodular graphite is somewhat higher than α cast iron with lamellar graphite.
Specific heat capacity of cast iron
The specific heat capacity of cast iron, like that of iron, increases with increasing temperature (see Table 2) and is characterized by an abrupt increase during the phase transformation Fe α → Fe λ ; then the specific heat cast iron drops sharply, but increases again with a further increase in temperature.
Graphitization lowers the specific heat capacity of cast iron; from here from white; cast iron is slightly higher than gray and high-strength cast iron (see Table 4).
Thermal conductivity of cast iron.
The thermal conductivity of cast iron is greater than others physical properties, depends on the structure, its dispersion and the smallest impurities, i.e., it is a structure-sensitive property.
Graphitization increases thermal conductivity; therefore, the elements that increase the degree of graphitization and the size of graphite increase, and the elements that prevent graphitization and increase the dispersion of structural components decrease. The indicated effect of graphitization is less for nodular graphite (see Table 4).
The shape of graphite, its precipitation and distribution also affect thermal conductivity. For example, ductile iron has a lower thermal conductivity than gray cast iron. The thermal conductivity of compacted graphite iron (CVG) is higher than that of compact graphite iron and is close to λ gray cast iron with lamellar graphite.
High-alloy cast irons are characterized, as a rule, by lower thermal conductivity than ordinary ones.